A change of PD-1/PD-L1 expression on peripheral T cell subsets correlates with the different stages of Alzheimer's Disease
- PMID: 36180897
- PMCID: PMC9524741
- DOI: 10.1186/s13578-022-00897-1
A change of PD-1/PD-L1 expression on peripheral T cell subsets correlates with the different stages of Alzheimer's Disease
Abstract
Background: Immune checkpoints are a set of costimulatory and inhibitory molecules that maintain self-tolerance and regulate immune homeostasis. The expression of immune checkpoints on T cells in malignancy, chronic inflammation, and neurodegenerative diseases has gained increasing attention.
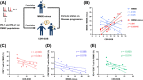
Results: To characterize immune checkpoints in neurodegenerative diseases, we aimed to examine the expression of the immune checkpoint PD-1/PD-L1 in peripheral T cells in different Alzheimer's disease (AD) patients. To achieve this aim, sixteen AD patients and sixteen age-matched healthy volunteers were enrolled to analyze their CD3+ T cells, CD3+CD56+ (neural cell adhesion molecule, NCAM) T cells, CD4+/CD8+ T cells, and CD4+/CD8+CD25+ (interleukin-2 receptor alpha, IL-2RA) T cells in this study. The expression of PD-1 on T cells was similar between the AD patients and healthy volunteers, but increased expression of PD-L1 on CD3+CD56+ T cells (natural killer T cells, NKT-like), CD4+ T cells (helper T cells, Th), CD4+CD25+ T cells, and CD8+ T cells (cytotoxic T lymphocytes, CTL) was detected in the AD patients. In addition, we found negative correlations between the AD patients' cognitive performance and both CD8+ T cells and CD8+CD25+ T cells. To identify CD8+ T-cell phenotypic and functional characteristic differences between the healthy volunteers and AD patients in different stages, a machine learning algorithm, t-distributed stochastic neighbor embedding (t-SNE), was implemented. Using t-SNE enabled the above high-dimensional data to be visualized and better analyzed. The t-SNE analysis demonstrated that the cellular sizes and densities of PD-1/PD-L1 on CD8+ T cells differed among the healthy, mild AD, and moderate AD subjects.
Conclusions: Our results suggest that changes in PD-1/PD-L1-expressing T cells in AD patients' peripheral blood could be a potential biomarker for monitoring disease and shed light on the AD disease mechanism. Moreover, these findings indicate that PD-1/PD-L1 blockade treatment could be a novel choice to slow AD disease deterioration.
Keywords: Alzheimer’s disease; Cognitive impairment; Immune checkpoint; Immunotherapy; PD-1, PD-L1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted without any commercial relationships that could be construed as potential conflicts of interest.
Figures





References
-
- Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, et al. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci USA. 1992;89(21):10075–10078. doi: 10.1073/pnas.89.21.10075. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

