Neutrophil β1 adrenoceptor blockade blunts stroke-associated neuroinflammation
- PMID: 36181002
- PMCID: PMC10100149
- DOI: 10.1111/bph.15963
Neutrophil β1 adrenoceptor blockade blunts stroke-associated neuroinflammation
Abstract
Background and purpose: Reperfusion therapy is the standard of care for ischaemic stroke; however, there is a need to identify new therapeutic targets able to ameliorate cerebral damage. Neutrophil β1 adrenoceptors (β1AR) have been linked to neutrophil migration during exacerbated inflammation. Given the central role of neutrophils in cerebral damage during stroke, we hypothesize that β1AR blockade will improve stroke outcomes.
Experimental approach: Rats were subjected to middle cerebral artery occlusion-reperfusion to evaluate the effect on stroke of the selective β1AR blocker metoprolol (12.5 mg·kg-1 ) when injected i.v. 10 min before reperfusion.
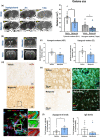
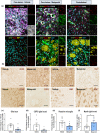
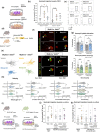
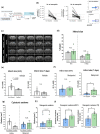
Key results: Magnetic resonance imaging and histopathology analysis showed that pre-reperfusion i.v. metoprolol reduced infarct size. This effect was accompanied by reduced cytotoxic oedema at 24 h and vasogenic oedema at 7 days. Metoprolol-treated rats showed reduced brain neutrophil infiltration and those which infiltrated displayed a high proportion of anti-inflammatory phenotype (N2, YM1+ ). Additional inflammatory models demonstrated that metoprolol specifically blocked neutrophil migration via β1AR and excluded a significant effect on the glia compartment. Consistently, metoprolol did not protect the brain in neutrophil-depleted rats upon stroke. In patients suffering an ischaemic stroke, β1AR blockade by metoprolol reduced circulating neutrophil-platelet co-aggregates.
Conclusions and implications: Our findings describe that β1AR blockade ameliorates cerebral damage by targeting neutrophils, identifying a novel therapeutic target to improve outcomes in patients with stroke. This therapeutic strategy is in the earliest stages of the translational pathway and should be further explored.
Keywords: Ischemic Stroke, Neutrophils, I/R, β1AR, Neuroinflammation.
© 2022 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
Javier Sanchez‐Gonzalez is an employee of Philips Healthcare (Madrid, Spain). All other authors have declared no conflict of interest.
Figures



References
-
- Ajmo, C. T. Jr. , Collier, L. A. , Leonardo, C. C. , Hall, A. A. , Green, S. M. , Womble, T. A. , Cuevas, J. , Willing, A. E. , & Pennypacker, K. R. (2009). Blockade of adrenoreceptors inhibits the splenic response to stroke. Experimental Neurology, 218(1), 47–55. 10.1016/j.expneurol.2009.03.044 - DOI - PMC - PubMed
-
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐Hosaini, K. , Bäck, M. , Barnes, N. M. , Bathgate, R. , … Ye, R. D. (2021). The concise guide to pharmacology 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(Suppl 1), S27–S156. - PubMed
-
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , Cirino, G. , Docherty, J. R. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Mangum, J. , Wonnacott, S. , & Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 - DOI - PMC - PubMed
-
- Ballesteros, I. , Rubio‐Ponce, A. , Genua, M. , Lusito, E. , Kwok, I. , Fernandez‐Calvo, G. , Khoyratty, T. E. , van Grinsven, E. , González‐Hernández, S. , Nicolás‐Ávila, J. Á. , Vicanolo, T. , Maccataio, A. , Benguría, A. , Li, J. L. , Adrover, J. M. , Aroca‐Crevillen, A. , Quintana, J. A. , Martín‐Salamanca, S. , Mayo, F. , … Hidalgo, A. (2020). Co‐option of neutrophil fates by tissue environments. Cell, 183(5), 1282–1297e1218. 10.1016/j.cell.2020.10.003 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

