Transfer of antibiotics and their metabolites in human milk: Implications for infant health and microbiota
- PMID: 36181712
- PMCID: PMC10763576
- DOI: 10.1002/phar.2732
Transfer of antibiotics and their metabolites in human milk: Implications for infant health and microbiota
Abstract
Antibiotics are an essential tool for perinatal care. While antibiotics can play a life-saving role for both parents and infants, they also cause collateral damage to the beneficial bacteria that make up the host gut microbiota. This is especially true for infants, whose developing gut microbiota is uniquely sensitive to antibiotic perturbation. Emerging evidence suggests that disruption of these bacterial populations during this crucial developmental window can have long-term effects on infant health and development. Although most current studies have focused on microbial disruptions caused by direct antibiotic administration to infants or prenatal exposure to antibiotics administered to the mother, little is known about whether antibiotics in human milk may pose similar risks to the infant. This review surveys current data on antibiotic transfer during lactation and highlights new methodologies to assess drug transfer in human milk. Finally, we provide recommendations for future work to ensure antibiotic use in lactating parents is safe and effective for both parents and infants.
Keywords: antibiotics; breastmilk; human milk; metabolomics.
© 2022 Pharmacotherapy Publications, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
PCD is an advisor to Cybele and co-founder and scientific advisor of Ometa and Enveda with prior approval by UC-San Diego. AHT is an advisor for Janssen Pharmaceuticals. CDC receives research funding from the following industry sponsors and a foundation: AstraZeneca; Celgene; GlaxoSmithKline; Janssen Pharmaceuticals; Pfizer, Inc.; Regeneron; Hoffman La-Roche-Genentech; Genzyme Sanofi-Aventis; Takeda Pharmaceutical Company Limited; Sanofi; UCB Pharma, USA; Sun Pharma Global FZE; Gilead; Novartis; and the Gerber Foundation. VN shares collaborative grants with Vaxcyte, Inc. and Cellics Therapeutics, and is a Scientific Advisor to ClaraMetyx Biosciences, Fortress Biotech, and SNIPR Biome.
Figures
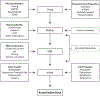

References
-
- Shorter E. Ignaz Semmelweis: the etiology, concept, and prophylaxis of childbed fever. Med Hist. 1984;28:334.
-
- Andrade SE, Gurwitz JH, Davis RL, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191:398–407. - PubMed
-
- Engeland A, Bjørge T, Klungsøyr K, Hjellvik V, Skurtveit S, Furu K. Trends in prescription drug use during pregnancy and postpartum in Norway, 2005 to 2015. Pharmacoepidemiol Drug Saf. 2018;27:995–1004. - PubMed

