Computational and functional studies of the PI(4,5)P2 binding site of the TRPM3 ion channel reveal interactions with other regulators
- PMID: 36181791
- PMCID: PMC9647539
- DOI: 10.1016/j.jbc.2022.102547
Computational and functional studies of the PI(4,5)P2 binding site of the TRPM3 ion channel reveal interactions with other regulators
Abstract
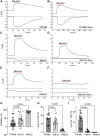
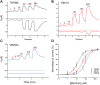
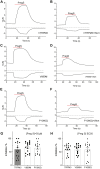
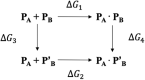
Transient receptor potential melastatin 3 (TRPM3) is a heat-activated ion channel expressed in peripheral sensory neurons and the central nervous system. TRPM3 activity depends on the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), but the molecular mechanism of activation by PI(4,5)P2 is not known. As no experimental structure of TRPM3 is available, we built a homology model of the channel in complex with PI(4,5)P2via molecular modeling. We identified putative contact residues for PI(4,5)P2 in the pre-S1 segment, the S4-S5 linker, and the proximal C-terminal TRP domain. Mutating these residues increased sensitivity to inhibition of TRPM3 by decreasing PI(4,5)P2 levels. Changes in ligand-binding affinities via molecular mechanics/generalized Born surface area (MM/GBSA) showed reduced PI(4,5)P2 affinity for the mutants. Mutating PI(4,5)P2-interacting residues also reduced sensitivity for activation by the endogenous ligand pregnenolone sulfate, pointing to an allosteric interaction between PI(4,5)P2 and pregnenolone sulfate. Similarly, mutating residues in the PI(4,5)P2 binding site in TRPM8 resulted in increased sensitivity to PI(4,5)P2 depletion and reduced sensitivity to menthol. Mutations of most PI(4,5)P2-interacting residues in TRPM3 also increased sensitivity to inhibition by Gβγ, indicating allosteric interaction between Gβγ and PI(4,5)P2 regulation. Disease-associated gain-of-function TRPM3 mutations on the other hand resulted in no change of PI(4,5)P2 sensitivity, indicating that mutations did not increase channel activity via increasing PI(4,5)P2 interactions. Our data provide insight into the mechanism of regulation of TRPM3 by PI(4,5)P2, its relationship to endogenous activators and inhibitors, as well as identify similarities and differences between PI(4,5)P2 regulation of TRPM3 and TRPM8.
Keywords: TRP channel; TRPM3; computational modeling; ion channel; phosphoinositide.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures






References
-
- Oberwinkler J., Philipp S.E. Trpm3. Handb Exp. Pharmacol. 2014;222:427–459. - PubMed
-
- Wagner T.F., Loch S., Lambert S., Straub I., Mannebach S., Mathar I., et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat. Cell Biol. 2008;10:1421–1430. - PubMed
-
- Straub I., Krugel U., Mohr F., Teichert J., Rizun O., Konrad M., et al. Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol. Pharmacol. 2013;84:736–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

