SARS-CoV-2 in immunocompromised individuals
- PMID: 36182669
- PMCID: PMC9468314
- DOI: 10.1016/j.immuni.2022.09.006
SARS-CoV-2 in immunocompromised individuals
Abstract
Immunocompromised individuals and particularly those with hematologic malignancies are at increased risk for SARS-CoV-2-associated morbidity and mortality due to immunologic deficits that limit prevention, treatment, and clearance of the virus. Understanding the natural history of viral infections in people with impaired immunity due to underlying conditions, immunosuppressive therapy, or a combination thereof has emerged as a critical area of investigation during the COVID-19 pandemic. Studies focused on these individuals have provided key insights into aspects of innate and adaptive immunity underlying both the antiviral immune response and excess inflammation in the setting of COVID-19. This review presents what is known about distinct states of immunologic vulnerability to SARS-CoV-2 and how this information can be harnessed to improve prevention and treatment strategies for immunologically high-risk populations.
Keywords: B cell; COVID-19; SARS-CoV-2; T cell; anti-CD20; cancer; immunocompromised; infection; vaccination.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.-A.P. reports honoraria from Abbvie, Astellas, Bristol-Myers Squibb, Celgene, Equilium, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, OrcaBio, Takeda, and VectivBio AG, Vor Biopharma. He serves on DSMBs for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier, and the scientific advisory board of NexImmune. He has ownership interests in NexImmune and Omeros. He has received research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, and Novartis. He serves in a volunteer capacity as a member of the Board of Directors of the American Society for Transplantation and Cellular Therapy (ASTCT) and Be The Match (National Marrow Donor Program, NMDP), as well as on the CIBMTR Cellular Immunotherapy Data Resource (CIDR) Executive Committee. M.V.D.B. has received research support and stock options from Seres Therapeutics and stock options from Notch Therapeutics and Pluto Therapeutics; he is a Scientific Co-founder and consultant for Thymofox, Inc; he has received royalties from Wolters Kluwer; has consulted, received honorarium from, or participated in advisory boards for Seres Therapeutics, WindMIL Therapeutics, Rheos Medicines, Merck & Co, Inc., Magenta Therapeutics, Frazier Healthcare Partners, Nektar Therapeutics, Notch Therapeutics, Forty Seven Inc., Ceramedix, Lygenesis, Pluto Therapeutics, GlaxoSmithKline, Da Volterra, Vor Biopharma, Novartis (Spouse), Synthekine (Spouse), and Beigene (Spouse); he has IP Licensing with Seres Therapeutics and Juno Therapeutics and holds a fiduciary role on the Foundation Board of DKMS (a nonprofit organization). S.V. is an advisor for Immunai and has provided consulting services for Koch Disruptive Technologies.
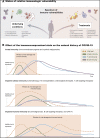
Figures

References
-
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R., Gurevich M. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. 17562864211012835. - DOI - PMC - PubMed
-
- Agbarya A., Sarel I., Ziv-Baran T., Agranat S., Schwartz O., Shai A., Nordheimer S., Fenig S., Shechtman Y., Kozlener E., et al. Efficacy of the mRNA-based BNT162b2 COVID-19 vaccine in patients with solid malignancies treated with anti-neoplastic drugs. Cancers. 2021;13:4191. doi: 10.3390/CANCERS13164191. - DOI - PMC - PubMed
-
- Andersen K.M., Bates B.A., Rashidi E.S., Olex A.L., Mannon R.B., Patel R.C., Singh J., Sun J., Auwaerter P.G., Ng D.K., et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet. Rheumatol. 2022;4:e33–e41. doi: 10.1016/S2665-9913(21)00325-8. - DOI - PMC - PubMed
-
- Angus D.C., Derde L., Al-Beidh F., Annane D., Arabi Y., Beane A., van Bentum-Puijk W., Berry L., Bhimani Z., Bonten M., et al. Effect of hydrocortisone on mortality and organ support in patients With severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. doi: 10.1001/JAMA.2020.17022. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

