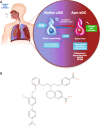
Inhaled mosliciguat (BAY 1237592): targeting pulmonary vasculature via activating apo-sGC
- PMID: 36183104
- PMCID: PMC9526466
- DOI: 10.1186/s12931-022-02189-1
Inhaled mosliciguat (BAY 1237592): targeting pulmonary vasculature via activating apo-sGC
Abstract
Background: Oxidative stress associated with severe cardiopulmonary diseases leads to impairment in the nitric oxide/soluble guanylate cyclase signaling pathway, shifting native soluble guanylate cyclase toward heme-free apo-soluble guanylate cyclase. Here we describe a new inhaled soluble guanylate cyclase activator to target apo-soluble guanylate cyclase and outline its therapeutic potential.
Methods: We aimed to generate a novel soluble guanylate cyclase activator, specifically designed for local inhaled application in the lung. We report the discovery and in vitro and in vivo characterization of the soluble guanylate cyclase activator mosliciguat (BAY 1237592).
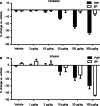
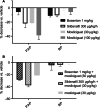
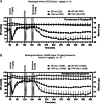
Results: Mosliciguat specifically activates apo-soluble guanylate cyclase leading to improved cardiopulmonary circulation. Lung-selective effects, e.g., reduced pulmonary artery pressure without reduced systemic artery pressure, were seen after inhaled but not after intravenous administration in a thromboxane-induced pulmonary hypertension minipig model. These effects were observed over a broad dose range with a long duration of action and were further enhanced under experimental oxidative stress conditions. In a unilateral broncho-occlusion minipig model, inhaled mosliciguat decreased pulmonary arterial pressure without ventilation/perfusion mismatch. With respect to airway resistance, mosliciguat showed additional beneficial bronchodilatory effects in an acetylcholine-induced rat model.
Conclusion: Inhaled mosliciguat may overcome treatment limitations in patients with pulmonary hypertension by improving pulmonary circulation and airway resistance without systemic exposure or ventilation/perfusion mismatch. Mosliciguat has the potential to become a new therapeutic paradigm, exhibiting a unique mode of action and route of application, and is currently under clinical development in phase Ib for pulmonary hypertension.
Keywords: Mosliciguat; Nitric oxide-insensitive soluble guanylate cyclase; Pulmonary diseases; Soluble guanylate cyclase activator; Ventilation/perfusion mismatch.
© 2022. The Author(s).
Conflict of interest statement
All authors are or were, at the time of the studies, employees of Bayer AG. J-PS, TK, and JS have retired since the studies were conducted.
In addition: EMB-P is mentioned as co-inventor on WO2014012934(A1) and on EP21218165.5 pending; FW is mentioned as co-inventor on WO2014012934(A1); MD is mentioned as co-inventor on WO2014012934(A1); JN is mentioned as co-inventor on EP21218165.5 pending; GW is mentioned as co-inventor on EP21218165.5 pending; J-PS is mentioned as co-inventor on WO2014012934(A1); LD is mentioned as co-inventor on EP21218165.5 pending; MGH is mentioned as co-inventor on WO2014012934(A1) and on EP21218165.5 pending.
Figures






References
-
- Adcirca (Tadalafil) US prescribing information. 2020. http://pi.lilly.com/us/adcirca-pi.pdf. Accessed Aug 2022.
-
- Revatio (Sildenafil) Highlights of US prescribing information. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021845s011,022.... Accessed Apr 2022.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

