Sex-differential non-specific effects of adjuvanted and non-adjuvanted rabies vaccines versus placebo on all-cause mortality in dogs (NERVE-Dog study): a study protocol for a randomized controlled trial with a nested case-control study
- PMID: 36183113
- PMCID: PMC9526991
- DOI: 10.1186/s12917-022-03455-6
Sex-differential non-specific effects of adjuvanted and non-adjuvanted rabies vaccines versus placebo on all-cause mortality in dogs (NERVE-Dog study): a study protocol for a randomized controlled trial with a nested case-control study
Abstract
Background: It has been proposed that childhood vaccines in high-mortality populations may have substantial impacts on mortality rates that are not explained by the prevention of targeted diseases, nor conversely by typical expected adverse reactions to the vaccines, and that these non-specific effects (NSEs) are generally more pronounced in females. The existence of these effects, and any implications for the development of vaccines and the design of vaccination programs to enhance safety, remain controversial. One area of controversy is the reported association of non-live vaccines with increased female mortality. In a previous randomized controlled trial (RCT), we observed that non-live alum-adjuvanted animal rabies vaccine (ARV) was associated with increased female but not male mortality in young, free-roaming dogs. Conversely, non-live non-adjuvanted human rabies vaccine (NRV) has been associated with beneficial non-specific effects in children. Alum adjuvant has been shown to suppress Th1 responses to pathogens, leading us to hypothesize that alum-adjuvanted rabies vaccine in young dogs has a detrimental effect on female survival by modulating the immune response to infectious and/or parasitic diseases. In this paper, we present the protocol of a 3-arm RCT comparing the effect of alum-adjuvanted rabies vaccine, non-adjuvanted rabies vaccine and placebo on all-cause mortality in an owned, free-roaming dog population, with causal mediation analysis of the RCT and a nested case-control study to test this hypothesis.
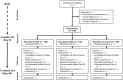
Methods: Randomised controlled trial with a nested case-control study.
Discussion: We expect that, among the placebo group, males will have higher mortality caused by higher pathogen loads and more severe disease, as determined by haematological parameters and inflammatory biomarkers. Among females, we expect that there will be no difference in mortality between the NRV and placebo groups, but that the ARV group will have higher mortality, again mediated by higher pathogen loads and more severe disease. We anticipate that these changes are preceded by shifts in key serum cytokine concentrations towards an anti-inflammatory immune response in females. If confirmed, these results will provide a rational basis for mitigation of detrimental NSEs of non-live vaccines in high-mortality populations.
Keywords: Cytokines; Dogs; Haemoparasites; Helminths; Mortality; Non-specific effects; Rabies; Sex; Vaccine; Viruses.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Sex-differential non-specific effects of rabies vaccine in dogs: An extended analysis of a randomized controlled trial in a high-mortality population.Vaccine. 2022 Mar 8;40(11):1674-1679. doi: 10.1016/j.vaccine.2021.01.029. Epub 2021 Jan 23. Vaccine. 2022. PMID: 33494967
-
β-Glucan as Trained Immunity-Based Adjuvants for Rabies Vaccines in Dogs.Front Immunol. 2020 Oct 8;11:564497. doi: 10.3389/fimmu.2020.564497. eCollection 2020. Front Immunol. 2020. PMID: 33162977 Free PMC article.
-
Population Dynamics of Owned, Free-Roaming Dogs: Implications for Rabies Control.PLoS Negl Trop Dis. 2015 Nov 6;9(11):e0004177. doi: 10.1371/journal.pntd.0004177. eCollection 2015 Nov. PLoS Negl Trop Dis. 2015. PMID: 26545242 Free PMC article.
-
Rabies control in Mexico.Dev Biol (Basel). 2008;131:167-75. Dev Biol (Basel). 2008. PMID: 18634477 Review.
-
The role of vaccination in rabies prevention.Curr Opin Virol. 2012 Jun;2(3):309-14. doi: 10.1016/j.coviro.2012.03.007. Epub 2012 Apr 11. Curr Opin Virol. 2012. PMID: 22503445 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical