Factors influencing acceptance, adoption and adherence to sentinel node biopsy recommendations in the Australian Melanoma Management Guidelines: a qualitative study using an implementation science framework
- PMID: 36183121
- PMCID: PMC9526940
- DOI: 10.1186/s43058-022-00351-w
Factors influencing acceptance, adoption and adherence to sentinel node biopsy recommendations in the Australian Melanoma Management Guidelines: a qualitative study using an implementation science framework
Abstract
Background: Sentinel node biopsy (SN biopsy) is a surgical procedure used to accurately stage patients with primary melanoma at high risk of recurrence. Although Australian Melanoma Management Guidelines recommend SN biopsy be considered in patients with melanomas > 1 mm thick, SN biopsy rates in Australia are reportedly low. Our objective was to identify factors impacting the acceptance, adoption and adherence to the Australian SN biopsy guideline recommendations.
Methods: Opinions of Australian key informants including clinicians, representatives from melanoma education and training providers, professional associations and colleges, and melanoma advocacy organisations were collected through semi-structured interviews (n = 29) and from publicly released statements (n = 14 news articles). Data analysis involved inductive and deductive thematic analysis using Flottorp's determinants framework.
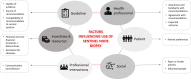
Results: A complex interplay of contemporary and historical factors was identified as influencing acceptance, adoption and adherence to the SN biopsy guideline recommendations at the individual, guideline, patient, organisational and social levels. Expert and peer opinion leaders have played an important role in facilitating or inhibiting adoption of guideline recommendations, as have financial incentives driven by healthcare-funding policies and non-financial incentives including professional identity and standing. Of critical importance have been the social and knowledge boundaries that exist between different professional groups to whom the guidelines apply (surgeons, dermatologists and primary care practitioners) with adherence to the guideline recommendations having the potential to shift work across professional boundaries, altering a clinician's workflow and revenue. More recently, the emergence of effective immunotherapies and targeted therapies for patients at high risk of recurrence, the emergence of new opinion leaders on the topic (in medical oncology), and patient demands for accurate staging are playing crucial roles in overcoming the resistance to change created by these social and knowledge boundaries.
Conclusions: Acceptance and adherence to SN biopsy guideline recommendations in Australia over the past 20 years has involved a process of renegotiation and reframing of the evidence for SN biopsy in melanoma by clinicians from different professional groups and networks. This process has helped to refine the evidence for SN biopsy and our understanding of appropriate adoption. New effective systemic therapies have changed the balance towards accepting guideline recommendations.
Keywords: Clinical practice guidelines; Implementation science framework; Melanoma; Professional groups; Sentinel lymph node biopsy.
© 2022. The Author(s).
Conflict of interest statement
RAS has received fees for professional services from F. Hoffmann-La Roche Ltd, Evaxion, Provectus Biopharmaceuticals Australia, Qbiotics, Novartis, Merck Sharp & Dohme, NeraCare, AMGEN Inc., Bristol-Myers Squibb, Myriad Genetics, GlaxoSmithKline.
GVL is a member of the executive committee for the Australian Clinical Practice Guidelines for the Diagnosis and Management of Melanoma. She is also a consultant advisor for Aduro Biotech Inc, Amgen Inc, Array Biopharma Inc, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Evaxion Biotech A/S, Hexel AG, Highlight Therapeutics S.L., Merck Sharpe & Dohme, Novartis Pharma AG, OncoSec, Pierre Fabre, QBiotics Group Limited, Regeneron Pharmaceuticals Inc, SkylineDX B.V., and Specialised Therapeutics Australia Pty Ltd.
RPMS has received honoraria for advisory board participation from MSD, Novartis and Qbiotics, speaking honoraria from BMS and Novartis and was a Working Party member of the current NHMRC Clinical Practice Guidelines for the Management of Cutaneous Melanoma (2018).
JFT has received honoraria for advisory board participation from BMS Australia, MSD Australia, GSK and Provectus Inc, and travel and conference support from GSK, Provectus Inc and Novartis.
Figures
Similar articles
-
Knowledge and attitudes of Australian dermatologists towards sentinel lymph node biopsy for melanoma: a mixed methods study.Australas J Dermatol. 2021 May;62(2):168-176. doi: 10.1111/ajd.13518. Epub 2020 Dec 5. Australas J Dermatol. 2021. PMID: 33277693
-
Effects of a regional guideline for completion axillary lymph node dissection in women with breast cancer to reduce variation in surgical practice: A qualitative study of physicians' views.Breast. 2016 Oct;29:126-31. doi: 10.1016/j.breast.2016.07.012. Epub 2016 Jul 30. Breast. 2016. PMID: 27484016
-
Sentinel Lymph Node Biopsy and Management of Regional Lymph Nodes in Melanoma: American Society of Clinical Oncology and Society of Surgical Oncology Clinical Practice Guideline Update.J Clin Oncol. 2018 Feb 1;36(4):399-413. doi: 10.1200/JCO.2017.75.7724. Epub 2017 Dec 12. J Clin Oncol. 2018. PMID: 29232171
-
Primary excision margins and sentinel lymph node biopsy in clinically node-negative melanoma of the trunk or extremities.Clin Oncol (R Coll Radiol). 2011 Nov;23(9):572-8. doi: 10.1016/j.clon.2011.04.012. Epub 2011 May 24. Clin Oncol (R Coll Radiol). 2011. PMID: 21605963
-
Role of sentinel lymph node biopsy as a staging procedure in patients with melanoma: A critical appraisal.Australas J Dermatol. 2017 Nov;58(4):268-273. doi: 10.1111/ajd.12655. Epub 2017 Jul 14. Australas J Dermatol. 2017. PMID: 28707391 Review.
References
-
- Australian Institute of Health and Welfare (AIHW). 2018 Cancer Data in Australia; Australian Cancer Incidence and Mortality (ACIM) books: melanoma of the skin. Canberra: AIHW 2018.
-
- Mahul AB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. Chicago: Springer International Publishing; 2017.
-
- Gershenwald JE, Scolyer RA. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Annals of Surgical Oncology 2018;25:2105–10. doi:10.1245/s10434-018-6513-7 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous