Fibroblast growth factor 10 attenuates chronic obstructive pulmonary disease by protecting against glycocalyx impairment and endothelial apoptosis
- PMID: 36183124
- PMCID: PMC9526324
- DOI: 10.1186/s12931-022-02193-5
Fibroblast growth factor 10 attenuates chronic obstructive pulmonary disease by protecting against glycocalyx impairment and endothelial apoptosis
Abstract
Background: The defects and imbalance in lung repair and structural maintenance contribute to the pathogenesis of chronic obstructive pulmonary diseases (COPD), yet the molecular mechanisms that regulate lung repair process are so far incompletely understood. We hypothesized that cigarette smoking causes glycocalyx impairment and endothelial apoptosis in COPD, which could be repaired by the stimulation of fibroblast growth factor 10 (FGF10)/FGF receptor 1 (FGFR1) signaling.
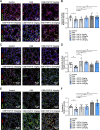
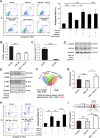
Methods: We used immunostaining (immunohistochemical [IHC] and immunofluorescence [IF]) and enzyme-linked immunosorbent assay (ELISA) to detect the levels of glycocalyx components and endothelial apoptosis in animal models and in patients with COPD. We used the murine emphysema model and in vitro studies to determine the protective and reparative role of FGF10/FGFR1.
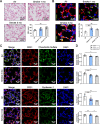
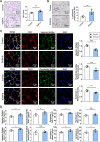
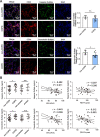
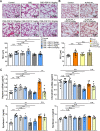
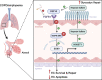
Results: Exposure to cigarette smoke caused endothelial glycocalyx impairment and emphysematous changes in murine models and human specimens. Pretreatment of FGF10 attenuated the development of emphysema and the shedding of glycocalyx components induced by CSE in vivo. However, FGF10 did not attenuate the emphysema induced by endothelial-specific killing peptide CGSPGWVRC-GG-D(KLAKLAK)2. Mechanistically, FGF10 alleviated smoke-induced endothelial apoptosis and glycocalyx repair through FGFR1/ERK/SOX9/HS6ST1 signaling in vitro. FGF10 was shown to repair pulmonary glycocalyx injury and endothelial apoptosis, and attenuate smoke-induced COPD through FGFR1 signaling.
Conclusions: Our results suggest that FGF10 may serve as a potential therapeutic strategy against COPD via endothelial repair and glycocalyx reconstitution.
Keywords: Chronic obstructive pulmonary diseases (COPD); Endothelial apoptosis; Fibroblast growth factor 10 (FGF10); Fibroblast growth factor receptor 1 (FGFR1); Glycocalyx.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Fibroblast growth factor 10 reverses cigarette smoke- and elastase-induced emphysema and pulmonary hypertension in mice.Eur Respir J. 2023 Nov 9;62(5):2201606. doi: 10.1183/13993003.01606-2022. Print 2023 Nov. Eur Respir J. 2023. PMID: 37884305 Free PMC article.
-
Fibroblast Growth Factor Signaling Mediates Pulmonary Endothelial Glycocalyx Reconstitution.Am J Respir Cell Mol Biol. 2017 Jun;56(6):727-737. doi: 10.1165/rcmb.2016-0338OC. Am J Respir Cell Mol Biol. 2017. PMID: 28187268 Free PMC article.
-
Pulmonary microvascular endothelial glycocalyx degradation as a key driver in COPD progression and its protection by Tongxinluo.Phytomedicine. 2025 Jul 25;143:156878. doi: 10.1016/j.phymed.2025.156878. Epub 2025 May 18. Phytomedicine. 2025. PMID: 40424984
-
Is alveolar destruction and emphysema in chronic obstructive pulmonary disease an immune disease?Proc Am Thorac Soc. 2006 Nov;3(8):687-90. doi: 10.1513/pats.200605-105SF. Proc Am Thorac Soc. 2006. PMID: 17065374 Review.
-
Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema.Respir Res. 2006 Mar 30;7(1):53. doi: 10.1186/1465-9921-7-53. Respir Res. 2006. PMID: 16571143 Free PMC article. Review.
Cited by
-
Study on the changes of extracellular matrix morphology and components in COPD animal model by using lung decellularized scaffold.FASEB J. 2025 Mar 31;39(6):e70463. doi: 10.1096/fj.202401522RR. FASEB J. 2025. PMID: 40150895 Free PMC article.
-
Evidence of premature vascular dysfunction in young adults who regularly use e-cigarettes and the impact of usage length.Angiogenesis. 2024 May;27(2):229-243. doi: 10.1007/s10456-023-09903-7. Epub 2024 Feb 12. Angiogenesis. 2024. PMID: 38345700 Free PMC article.
-
Factors Influencing the Maturation and Developmental Competence of Yak (Bos grunniens) Oocytes In Vitro.Genes (Basel). 2023 Sep 27;14(10):1882. doi: 10.3390/genes14101882. Genes (Basel). 2023. PMID: 37895231 Free PMC article. Review.
-
The endothelial glycocalyx-All the same? No, it is not.Acute Med Surg. 2023 Oct 4;10(1):e896. doi: 10.1002/ams2.896. eCollection 2023 Jan-Dec. Acute Med Surg. 2023. PMID: 37808968 Free PMC article. Review.
-
Protection against cigarette smoke-induced chronic obstructive pulmonary disease via activation of the SIRT1/FoxO1 axis by targeting microRNA-132.Am J Transl Res. 2024 Oct 15;16(10):5516-5524. doi: 10.62347/FVQP4019. eCollection 2024. Am J Transl Res. 2024. PMID: 39544778 Free PMC article.
References
-
- Collaborators GBDCRD Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. - DOI - PMC - PubMed
-
- Haeger SM, Liu X, Han X, McNeil JB, Oshima K, McMurtry SA, Yang Y, Ouyang Y, Zhang F, Nozik-Grayck E, et al. Epithelial heparan sulfate contributes to alveolar barrier function and is shed during lung injury. Am J Respir Cell Mol Biol. 2018;59:363–374. doi: 10.1165/rcmb.2017-0428OC. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

