Improving the safety and experience of transitions from hospital to home: a cluster randomised controlled feasibility trial of the 'Your Care Needs You' intervention versus usual care
- PMID: 36183129
- PMCID: PMC9525931
- DOI: 10.1186/s40814-022-01180-3
Improving the safety and experience of transitions from hospital to home: a cluster randomised controlled feasibility trial of the 'Your Care Needs You' intervention versus usual care
Abstract
Background: The 'Your Care Needs You' (YCNY) intervention aims to increase the safety and experience of transitions for older people through greater patient involvement during the hospital stay.
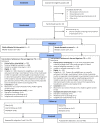
Methods: A cluster randomised controlled feasibility trial was conducted on NHS inpatient wards (clusters) where ≥ 40% of patients were routinely ≥ 75 years. Wards were randomised to YCNY or usual care using an unequal allocation ratio (3:2). We aimed to recruit up to 20 patients per ward. Follow-up included routine data collection and questionnaires at 5-, 30-, and 90-days post-discharge. Eligible patients were ≥ 75 years, discharged home, stayed overnight on participating wards, and could read and understand English. The trial assessed the feasibility of delivering YCNY and the trial methodology through recruitment rates, outcome completion rates, and a qualitative evaluation. The accuracy of using routinely coded data for the primary outcome in the definitive trial was assessed by extracting discharge information for up to ten nonindividual consenting patients per ward.
Results: Ten wards were randomised (6 intervention, 4 control). One ward withdrew, and two wards were unable to deliver the intervention. Seven-hundred twenty-one patients were successfully screened, and 161 were recruited (95 intervention, 66 control). The patient post-discharge attrition rate was 17.4% (n = 28). Primary outcome data were gathered for 91.9% of participants with 75.2% and 59.0% providing secondary outcome data at 5 and 30 days post-discharge respectively. Item completion within questionnaires was generally high. Post-discharge follow-up was terminated early due to the COVID-19 pandemic affecting 90-day response rates (16.8%). Data from 88 nonindividual consenting patients identified an error rate of 15% when using routinely coded data for the primary outcome. No unexpected serious adverse events were identified. Most patients viewed YCNY favourably. Staff agreed with it in principle, but ward pressures and organisational contexts hampered implementation. There was a need to sustain engagement, provide clarity on roles and responsibilities, and account for fluctuations in patients' health, capacity, and preferences.
Conclusions: If implementation challenges can be overcome, YCNY represents a step towards involving older people as partners in their care to improve the safety and experience of their transitions from hospital to home.
Trial registration: ISRCTN: 51154948.
Keywords: Care of older people; Cluster randomised controlled trial; Complex intervention; Feasibility trial; Hospital discharge; Patient experience; Patient safety; Transitions of care.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Evaluating an intervention to improve the safety and experience of transitions from hospital to home for older people (Your Care Needs You): a protocol for a cluster randomised controlled trial and process evaluation.Trials. 2023 Oct 14;24(1):671. doi: 10.1186/s13063-023-07716-z. Trials. 2023. PMID: 37838678 Free PMC article.
-
Improving patient experience and safety at transitions of care through the Your Care Needs You (YCNY) intervention: a study protocol for a cluster randomised controlled feasibility trial.Pilot Feasibility Stud. 2020 Sep 2;6:123. doi: 10.1186/s40814-020-00655-5. eCollection 2020. Pilot Feasibility Stud. 2020. PMID: 32905158 Free PMC article.
-
Understanding the challenges and successes of implementing 'hybrid' interventions in healthcare settings: findings from a process evaluation of a patient involvement trial.BMJ Qual Saf. 2025 Jan 28;34(2):92-99. doi: 10.1136/bmjqs-2024-017268. BMJ Qual Saf. 2025. PMID: 39107110 Free PMC article. Clinical Trial.
-
Impact of summer programmes on the outcomes of disadvantaged or 'at risk' young people: A systematic review.Campbell Syst Rev. 2024 Jun 13;20(2):e1406. doi: 10.1002/cl2.1406. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38873396 Free PMC article. Review.
-
Cue-based versus scheduled feeding for preterm infants transitioning from tube to oral feeding: the Cubs mixed-methods feasibility study.Health Technol Assess. 2021 Dec;25(74):1-146. doi: 10.3310/hta25740. Health Technol Assess. 2021. PMID: 34878383
Cited by
-
Improving the safety and experience of transitions from hospital to home: a cluster randomised controlled trial of an intervention to involve older people in their care (Your Care Needs You).Age Ageing. 2025 May 3;54(5):afaf142. doi: 10.1093/ageing/afaf142. Age Ageing. 2025. PMID: 40444747 Free PMC article. Clinical Trial.
-
Evaluating an intervention to improve the safety and experience of transitions from hospital to home for older people (Your Care Needs You): a protocol for a cluster randomised controlled trial and process evaluation.Trials. 2023 Oct 14;24(1):671. doi: 10.1186/s13063-023-07716-z. Trials. 2023. PMID: 37838678 Free PMC article.
References
-
- NHS Digital. Emergency readmissions to hospital within 30 days of discharge: indirectly standardised percent trends broken down by age bands and sex. 2022. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/com.... Cited 2022 June 13.
-
- van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. 2012;18(6):211–218. - PubMed
-
- Yam CH, Wong EL, Chan FW, Wong FY, Leung MC, Yeoh EK. Measuring and preventing potentially avoidable hospital readmissions: a review of the literature. Hong Kong Med J. 2010;16(5):383–389. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical