Combination of long-term 13CO2 labeling and isotopolog profiling allows turnover analysis of photosynthetic pigments in Arabidopsis leaves
- PMID: 36183136
- PMCID: PMC9526918
- DOI: 10.1186/s13007-022-00946-3
Combination of long-term 13CO2 labeling and isotopolog profiling allows turnover analysis of photosynthetic pigments in Arabidopsis leaves
Abstract
Background: Living cells maintain and adjust structural and functional integrity by continual synthesis and degradation of metabolites and macromolecules. The maintenance and adjustment of thylakoid membrane involve turnover of photosynthetic pigments along with subunits of protein complexes. Quantifying their turnover is essential to understand the mechanisms of homeostasis and long-term acclimation of photosynthetic apparatus. Here we report methods combining whole-plant long-term 13CO2 labeling and liquid chromatography - mass spectrometry (LC-MS) analysis to determine the size of non-labeled population (NLP) of carotenoids and chlorophylls (Chl) in leaf pigment extracts of partially 13C-labeled plants.
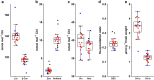
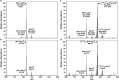
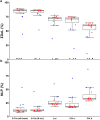
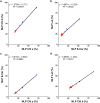
Results: The labeling chamber enabled parallel 13CO2 labeling of up to 15 plants of Arabidopsis thaliana with real-time environmental monitoring ([CO2], light intensity, temperature, relative air humidity and pressure) and recording. No significant difference in growth or photosynthetic pigment composition was found in leaves after 7-d exposure to normal CO2 (~ 400 ppm) or 13CO2 in the labeling chamber, or in ambient air outside the labeling chamber (control). Following chromatographic separation of the pigments and mass peak assignment by high-resolution Fourier-transform ion cyclotron resonance MS, mass spectra of photosynthetic pigments were analyzed by triple quadrupole MS to calculate NLP. The size of NLP remaining after the 7-d 13CO2 labeling was ~ 10.3% and ~ 11.5% for all-trans- and 9-cis-β-carotene, ~ 21.9% for lutein, ~ 18.8% for Chl a and 33.6% for Chl b, highlighting non-uniform turnover of these pigments in thylakoids. Comparable results were obtained in all replicate plants of the 13CO2 labeling experiment except for three that were showing anthocyanin accumulation and growth impairment due to insufficient water supply (leading to stomatal closure and less 13C incorporation).
Conclusions: Our methods allow 13CO2 labeling and estimation of NLP for photosynthetic pigments with high reproducibility despite potential variations in [13CO2] between the experiments. The results indicate distinct turnover rates of carotenoids and Chls in thylakoid membrane, which can be investigated in the future by time course experiments. Since 13C enrichment can be measured in a range of compounds, long-term 13CO2 labeling chamber, in combination with appropriate MS methods, facilitates turnover analysis of various metabolites and macromolecules in plants on a time scale of hours to days.
Keywords: 13CO2; Carotene; Carotenoids; Chlorophyll; Lutein; Pigment metabolism; Stable isotope labeling; Turnover.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis revealed by 14CO2 pulse-chase labeling.Plant Physiol. 2010 Apr;152(4):2188-99. doi: 10.1104/pp.109.151647. Epub 2010 Jan 29. Plant Physiol. 2010. PMID: 20118270 Free PMC article.
-
Carotenoid Isotopolog Profiling in 13C-Labeled Leaf Extracts by LC-MS and LC-FTICR-MS.Methods Mol Biol. 2020;2083:263-277. doi: 10.1007/978-1-4939-9952-1_20. Methods Mol Biol. 2020. PMID: 31745928
-
Altered turnover of β-carotene and Chl a in Arabidopsis leaves treated with lincomycin or norflurazon.Plant Cell Physiol. 2011 Jul;52(7):1193-203. doi: 10.1093/pcp/pcr069. Epub 2011 Jun 1. Plant Cell Physiol. 2011. PMID: 21632655
-
Biosynthesis, accumulation and emission of carotenoids, alpha-tocopherol, plastoquinone, and isoprene in leaves under high photosynthetic irradiance.Photosynth Res. 2007 May;92(2):163-79. doi: 10.1007/s11120-007-9204-y. Epub 2007 Jul 17. Photosynth Res. 2007. PMID: 17634750 Review.
-
Spectral and kinetic parameters of phosphorescence of triplet chlorophyll a in the photosynthetic apparatus of plants.Biochemistry (Mosc). 2014 Apr;79(4):349-61. doi: 10.1134/S000629791404004X. Biochemistry (Mosc). 2014. PMID: 24910208 Review.
References
-
- Penning de Vries FWT The cost of maintenance processes in plant cells. Ann Bot. 1975;39:77–92. doi: 10.1093/oxfordjournals.aob.a084919. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

