Skin fibrosis associated with keloid, scleroderma and Jorge Lobo's disease (lacaziosis): An immuno-histochemical study
- PMID: 36183172
- PMCID: PMC9664412
- DOI: 10.1111/iep.12456
Skin fibrosis associated with keloid, scleroderma and Jorge Lobo's disease (lacaziosis): An immuno-histochemical study
Abstract
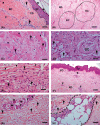
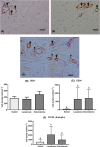
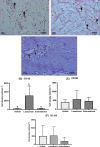
Fibrosis is a common pathophysiological response of many tissues and organs subjected to chronic injury. Despite the diverse aetiology of keloid, lacaziosis and localized scleroderma, the process of fibrosis is present in the pathogenesis of all of these three entities beyond other individual clinical and histological distinct characteristics. Fibrosis was studied in 20 samples each of these three chronic cutaneous inflammatory diseases. An immunohistochemical study was carried out to explore the presence of α-smooth muscle actin (α-SMA) and vimentin cytoskeleton antigens, CD31, CD34, Ki67, p16; CD105, CD163, CD206 and FOXP3 antigens; and the central fibrotic cytokine TGF-β. Higher expression of vimentin in comparison to α-SMA in all three lesion types was found. CD31- and CD34-positive blood vessel endothelial cells were observed throughout the reticular dermis. Ki67 expression was low and almost absent in scleroderma. p16-positive levels were higher than ki67 and observed in reticular dermis of keloidal collagen in keloids, in collagen bundles in scleroderma and in the external layers of the granulomas in lacaziosis. The presence of α-actin positive cells and rarely CD34 positive cells, observed primarily in keloids, may be related to higher p16 antigen expression, a measure of cell senescence. Low FOXP3 expression was observed in all lesion types. CD105-positive cells were mainly found in perivascular tissue in close contact with the adventitia in keloids and scleroderma, while, in lacaziosis, these cells were chiefly observed in conjunction with collagen deposition in the external granuloma layer. We did not find high involvement of CD163 or CD206-positive cells in the fibrotic process. TGF-β was notable only in keloid and lacaziosis lesions. In conclusion, we have suggested vimentin to be the main myofibroblast general marker of the fibrotic process in all three studied diseases, while endothelial-to-mesenchymal transition (EndoMT) and mesenchymal stem cells (MSCs) and M2 macrophages may not play an important role.
Keywords: fibrosis; immunohistochemistry; keloid; lacaziosis; scleroderma.
© 2022 Company of the International Journal of Experimental Pathology (CIJEP).
Conflict of interest statement
The authors declare no conflict of interest in this work.
Figures


Similar articles
-
Hypertrophic and keloid scars fail to progress from the CD34- /α-smooth muscle actin (α-SMA)+ immature scar phenotype and show gradient differences in α-SMA and p16 expression.Br J Dermatol. 2020 Apr;182(4):974-986. doi: 10.1111/bjd.18219. Epub 2019 Sep 4. Br J Dermatol. 2020. PMID: 31206605
-
Keloid-like scleroderma.Am J Dermatopathol. 2003 Aug;25(4):327-30. doi: 10.1097/00000372-200308000-00007. Am J Dermatopathol. 2003. PMID: 12876490
-
Connective tissue growth factor gene expression in tissue sections from localized scleroderma, keloid, and other fibrotic skin disorders.J Invest Dermatol. 1996 Apr;106(4):729-33. doi: 10.1111/1523-1747.ep12345771. J Invest Dermatol. 1996. PMID: 8618012
-
Keloid disorder: Fibroblast differentiation and gene expression profile in fibrotic skin diseases.Exp Dermatol. 2021 Jan;30(1):132-145. doi: 10.1111/exd.14243. Epub 2020 Dec 20. Exp Dermatol. 2021. PMID: 33211348 Review.
-
Jorge Lobo's disease: a case of keloidal blastomycosis (lobomycosis) in a nonendemic area.Ther Adv Infect Dis. 2014 Jun;2(3-4):91-6. doi: 10.1177/2049936114559919. Ther Adv Infect Dis. 2014. PMID: 25469235 Free PMC article. Review.
Cited by
-
Biomarkers in skin autoimmunity-An update on localised scleroderma.Skin Health Dis. 2024 Jan 12;4(2):e335. doi: 10.1002/ski2.335. eCollection 2024 Apr. Skin Health Dis. 2024. PMID: 38577035 Free PMC article. Review.
References
-
- Friedman SL. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract. 2004;1:98‐105. - PubMed
-
- Distler JHW, Györfi AH, Ramanujam M, Whitfield ML, Königshoff M, Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. 2019;15:705‐730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

