Atypical induction of HIF-1α expression by pericellular Notch1 signaling suffices for the malignancy of glioblastoma multiforme cells
- PMID: 36183290
- PMCID: PMC9527190
- DOI: 10.1007/s00018-022-04529-2
Atypical induction of HIF-1α expression by pericellular Notch1 signaling suffices for the malignancy of glioblastoma multiforme cells
Abstract
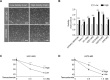
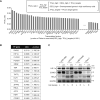
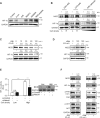
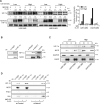
Contact-based pericellular interactions play important roles in cancer progression via juxtacrine signaling pathways. The present study revealed that hypoxia-inducible factor-1α (HIF-1α), induced even in non-hypoxic conditions by cell-to-cell contact, was a critical cue responsible for the malignant characteristics of glioblastoma multiforme (GBM) cells through Notch1 signaling. Densely cultured GBM cells showed enhanced viability and resistance to temozolomide (TMZ) compared to GBM cells at a low density. Ablating Notch1 signaling by a γ-secretase inhibitor or siRNA transfection resensitized resistant GBM cells to TMZ treatment and decreased their viability under dense culture conditions. The expression of HIF-1α was significantly elevated in highly dense GBM cells even under non-hypoxic conditions. Atypical HIF-1α expression was associated with the Notch1 signaling pathway in both GBM and glioblastoma stem cells (GSC). Proteasomal degradation of HIF-1α was prevented by binding with Notch1 intracellular domain (NICD), which translocated to the nuclei of GBM cells. Silencing Notch1 signaling using a doxycycline-inducible Notch1 RNA-interfering system or treatment with chetomin, a HIF pathway inhibitor, retarded tumor development with a significant anti-cancer effect in a murine U251-xenograft model. Using GBM patient tissue microarray analysis, a significant increase in HIF-1α expression was identified in the group with Notch1 expression compared to the group without Notch1 expression among those with positive HIF-1α expression. Collectively, these findings highlight the critical role of cell-to-cell contact-dependent signaling in GBM progression. They provide a rationale for targeting HIF-1α signaling even in a non-hypoxic microenvironment.
Keywords: Abnormal HIF-1α; Chemoresistance; GBM; Juxtacrine signaling; Notch1.
© 2022. The Author(s).
Conflict of interest statement
CC is the founder and shareholder of ILIAS Biologics, Inc. EK and KChoi are minor shareholders of ILIAS Biologics, Inc. The other authors declare that they have no competing interests.
Figures


Similar articles
-
Enhancing temozolomide efficacy in GBM: The synergistic role of chuanxiong rhizoma essential oil.Phytomedicine. 2025 May;140:156575. doi: 10.1016/j.phymed.2025.156575. Epub 2025 Mar 10. Phytomedicine. 2025. PMID: 40088740
-
Hypoxia enhances IL-8 signaling through inhibiting miR-128-3p expression in glioblastomas.Biochim Biophys Acta Mol Cell Res. 2025 Feb;1872(2):119885. doi: 10.1016/j.bbamcr.2024.119885. Epub 2024 Dec 2. Biochim Biophys Acta Mol Cell Res. 2025. PMID: 39631468
-
Propofol enhances the sensitivity of glioblastoma cells to temozolomide by inhibiting macrophage activation in tumor microenvironment to down-regulate HIF-1α expression.Exp Cell Res. 2022 Sep 15;418(2):113277. doi: 10.1016/j.yexcr.2022.113277. Epub 2022 Jul 8. Exp Cell Res. 2022. PMID: 35810776
-
Advances in the targeting of HIF-1α and future therapeutic strategies for glioblastoma multiforme (Review).Oncol Rep. 2017 Feb;37(2):657-670. doi: 10.3892/or.2016.5309. Epub 2016 Dec 9. Oncol Rep. 2017. PMID: 27959421 Review.
-
Hypoxia: The Cornerstone of Glioblastoma.Int J Mol Sci. 2021 Nov 22;22(22):12608. doi: 10.3390/ijms222212608. Int J Mol Sci. 2021. PMID: 34830491 Free PMC article. Review.
Cited by
-
Deciphering the role of transcription factors in glioblastoma cancer stem cells.Acta Biochim Biophys Sin (Shanghai). 2024 May 8;56(9):1245-1255. doi: 10.3724/abbs.2024061. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38716541 Free PMC article. Review.
-
Adult IDH Wild-Type Glioblastoma Ultrastructural Investigation Suggests a Possible Correlation between Morphological Biomarkers and Ki-67 Index.Biomedicines. 2023 Jul 12;11(7):1968. doi: 10.3390/biomedicines11071968. Biomedicines. 2023. PMID: 37509607 Free PMC article.
-
Tumor-suppressive function and mechanism of miR-873-5p in glioblastoma: evidence based on bioinformatics analysis and experimental validation.Aging (Albany NY). 2023 Jun 28;15(12):5412-5425. doi: 10.18632/aging.204800. Epub 2023 Jun 28. Aging (Albany NY). 2023. PMID: 37382594 Free PMC article.
-
PCSK5 downregulation promotes the inhibitory effect of andrographolide on glioblastoma through regulating STAT3.Mol Cell Biochem. 2025 Jan;480(1):521-533. doi: 10.1007/s11010-024-04977-3. Epub 2024 Mar 29. Mol Cell Biochem. 2025. PMID: 38553549
-
HIF-1α Mediated Regulation of Glioblastoma Malignant Phenotypes through CD47 Protein: Understanding Functions and Mechanisms.J Cancer. 2025 Jan 1;16(3):750-764. doi: 10.7150/jca.101050. eCollection 2025. J Cancer. 2025. PMID: 39781344 Free PMC article.
References
-
- Oshiro S, Tsugu H, Komatsu F, Ohmura T, Ohta M, Sakamoto S, Fukushima T, Inoue T. Efficacy of temozolomide treatment in patients with high-grade glioma. Anticancer Res. 2009;29:911–917. - PubMed
-
- Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, Fink K, Robins HI, De Angelis L, Raizer J, Hess K, Aldape K, Lamborn KR, Kuhn J, Dancey J, Prados MD, C. North American Brain Tumor and I. the National Cancer Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Investig New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

