Access and utilization of long chain fatty acyl-CoA by zDHHC protein acyltransferases
- PMID: 36183446
- PMCID: PMC9772126
- DOI: 10.1016/j.sbi.2022.102463
Access and utilization of long chain fatty acyl-CoA by zDHHC protein acyltransferases
Abstract
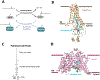
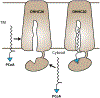
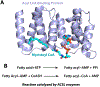
S-acylation is a reversible posttranslational modification, where a long-chain fatty acid is attached to a protein through a thioester linkage. Being the most abundant form of lipidation in humans, a family of twenty-three human zDHHC integral membrane enzymes catalyze this reaction. Previous structures of the apo and lipid bound zDHHCs shed light into the molecular details of the active site and binding pocket. Here, we delve further into the details of fatty acyl-CoA recognition by zDHHC acyltransferases using insights from the recent structure. We additionally review indirect evidence that suggests acyl-CoAs do not diffuse freely in the cytosol, but are channeled into specific pathways, and comment on the suggested mechanisms for fatty acyl-CoA compartmentalization and intracellular transport, to finally speculate about the potential mechanisms that underlie fatty acyl-CoA delivery to zDHHC enzymes.
Published by Elsevier Ltd.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures
Similar articles
-
The molecular mechanism of DHHC protein acyltransferases.Biochem Soc Trans. 2019 Feb 28;47(1):157-167. doi: 10.1042/BST20180429. Epub 2018 Dec 17. Biochem Soc Trans. 2019. PMID: 30559274 Review.
-
Molecular basis of fatty acid selectivity in the zDHHC family of S-acyltransferases revealed by click chemistry.Proc Natl Acad Sci U S A. 2017 Feb 21;114(8):E1365-E1374. doi: 10.1073/pnas.1612254114. Epub 2017 Feb 6. Proc Natl Acad Sci U S A. 2017. PMID: 28167757 Free PMC article.
-
Bivalent recognition of fatty acyl-CoA by a human integral membrane palmitoyltransferase.Proc Natl Acad Sci U S A. 2022 Feb 15;119(7):e2022050119. doi: 10.1073/pnas.2022050119. Proc Natl Acad Sci U S A. 2022. PMID: 35140179 Free PMC article.
-
A facile assay for zDHHC palmitoyl transferase activation elucidates effects of mutation and modification.J Lipid Res. 2025 Feb;66(2):100743. doi: 10.1016/j.jlr.2025.100743. Epub 2025 Jan 10. J Lipid Res. 2025. PMID: 39800157 Free PMC article.
-
Regulatory effects of post-translational modifications on zDHHC S-acyltransferases.J Biol Chem. 2020 Oct 23;295(43):14640-14652. doi: 10.1074/jbc.REV120.014717. Epub 2020 Aug 17. J Biol Chem. 2020. PMID: 32817054 Free PMC article. Review.
Cited by
-
CellPalmSeq: A curated RNAseq database of palmitoylating and de-palmitoylating enzyme expression in human cell types and laboratory cell lines.Front Physiol. 2023 Jan 24;14:1110550. doi: 10.3389/fphys.2023.1110550. eCollection 2023. Front Physiol. 2023. PMID: 36760531 Free PMC article.
-
Mechanisms and functions of protein S-acylation.Nat Rev Mol Cell Biol. 2024 Jun;25(6):488-509. doi: 10.1038/s41580-024-00700-8. Epub 2024 Feb 14. Nat Rev Mol Cell Biol. 2024. PMID: 38355760 Free PMC article. Review.
-
In vitro reconstitution reveals substrate selectivity of protein S-acyltransferases.J Biol Chem. 2025 Apr;301(4):108406. doi: 10.1016/j.jbc.2025.108406. Epub 2025 Mar 14. J Biol Chem. 2025. PMID: 40090582 Free PMC article.
-
The role of an amphiphilic helix and transmembrane region in the efficient acylation of the M2 protein from influenza virus.Sci Rep. 2023 Nov 2;13(1):18928. doi: 10.1038/s41598-023-45945-z. Sci Rep. 2023. PMID: 37919373 Free PMC article.
-
Altered Protein Palmitoylation as Disease Mechanism in Neurodegenerative Disorders.J Neurosci. 2024 Oct 2;44(40):e1225242024. doi: 10.1523/JNEUROSCI.1225-24.2024. J Neurosci. 2024. PMID: 39358031 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

