Traumatic brain injury: progress and challenges in prevention, clinical care, and research
- PMID: 36183712
- PMCID: PMC10427240
- DOI: 10.1016/S1474-4422(22)00309-X
Traumatic brain injury: progress and challenges in prevention, clinical care, and research
Erratum in
-
Correction to Lancet Neurol 2022; published online Sept 29. https://doi.org/10.1016/ S1474-4422(22)00309-X.Lancet Neurol. 2022 Dec;21(12):e10. doi: 10.1016/S1474-4422(22)00411-2. Epub 2022 Oct 7. Lancet Neurol. 2022. PMID: 36216016 No abstract available.
Abstract
Traumatic brain injury (TBI) has the highest incidence of all common neurological disorders, and poses a substantial public health burden. TBI is increasingly documented not only as an acute condition but also as a chronic disease with long-term consequences, including an increased risk of late-onset neurodegeneration. The first Lancet Neurology Commission on TBI, published in 2017, called for a concerted effort to tackle the global health problem posed by TBI. Since then, funding agencies have supported research both in high-income countries (HICs) and in low-income and middle-income countries (LMICs). In November 2020, the World Health Assembly, the decision-making body of WHO, passed resolution WHA73.10 for global actions on epilepsy and other neurological disorders, and WHO launched the Decade for Action on Road Safety plan in 2021. New knowledge has been generated by large observational studies, including those conducted under the umbrella of the International Traumatic Brain Injury Research (InTBIR) initiative, established as a collaboration of funding agencies in 2011. InTBIR has also provided a huge stimulus to collaborative research in TBI and has facilitated participation of global partners. The return on investment has been high, but many needs of patients with TBI remain unaddressed. This update to the 2017 Commission presents advances and discusses persisting and new challenges in prevention, clinical care, and research.
In LMICs, the occurrence of TBI is driven by road traffic incidents, often involving vulnerable road users such as motorcyclists and pedestrians. In HICs, most TBI is caused by falls, particularly in older people (aged ≥65 years), who often have comorbidities. Risk factors such as frailty and alcohol misuse provide opportunities for targeted prevention actions. Little evidence exists to inform treatment of older patients, who have been commonly excluded from past clinical trials—consequently, appropriate evidence is urgently required. Although increasing age is associated with worse outcomes from TBI, age should not dictate limitations in therapy. However, patients injured by low-energy falls (who are mostly older people) are about 50% less likely to receive critical care or emergency interventions, compared with those injured by high-energy mechanisms, such as road traffic incidents.
Mild TBI, defined as a Glasgow Coma sum score of 13–15, comprises most of the TBI cases (over 90%) presenting to hospital. Around 50% of adult patients with mild TBI presenting to hospital do not recover to pre-TBI levels of health by 6 months after their injury. Fewer than 10% of patients discharged after presenting to an emergency department for TBI in Europe currently receive follow-up. Structured follow-up after mild TBI should be considered good practice, and urgent research is needed to identify which patients with mild TBI are at risk for incomplete recovery.
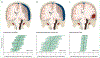
The selection of patients for CT is an important triage decision in mild TBI since it allows early identification of lesions that can trigger hospital admission or life-saving surgery. Current decision making for deciding on CT is inefficient, with 90–95% of scanned patients showing no intracranial injury but being subjected to radiation risks. InTBIR studies have shown that measurement of blood-based biomarkers adds value to previously proposed clinical decision rules, holding the potential to improve efficiency while reducing radiation exposure. Increased concentrations of biomarkers in the blood of patients with a normal presentation CT scan suggest structural brain damage, which is seen on MR scanning in up to 30% of patients with mild TBI. Advanced MRI, including diffusion tensor imaging and volumetric analyses, can identify additional injuries not detectable by visual inspection of standard clinical MR images. Thus, the absence of CT abnormalities does not exclude structural damage—an observation relevant to litigation procedures, to management of mild TBI, and when CT scans are insufficient to explain the severity of the clinical condition.
Although blood-based protein biomarkers have been shown to have important roles in the evaluation of TBI, most available assays are for research use only. To date, there is only one vendor of such assays with regulatory clearance in Europe and the USA with an indication to rule out the need for CT imaging for patients with suspected TBI. Regulatory clearance is provided for a combination of biomarkers, although evidence is accumulating that a single biomarker can perform as well as a combination. Additional biomarkers and more clinical-use platforms are on the horizon, but cross-platform harmonisation of results is needed. Health-care efficiency would benefit from diversity in providers.
In the intensive care setting, automated analysis of blood pressure and intracranial pressure with calculation of derived parameters can help individualise management of TBI. Interest in the identification of subgroups of patients who might benefit more from some specific therapeutic approaches than others represents a welcome shift towards precision medicine. Comparative-effectiveness research to identify best practice has delivered on expectations for providing evidence in support of best practices, both in adult and paediatric patients with TBI.
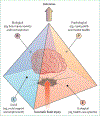
Progress has also been made in improving outcome assessment after TBI. Key instruments have been translated into up to 20 languages and linguistically validated, and are now internationally available for clinical and research use. TBI affects multiple domains of functioning, and outcomes are affected by personal characteristics and life-course events, consistent with a multifactorial bio-psycho-socio-ecological model of TBI, as presented in the US National Academies of Sciences, Engineering, and Medicine (NASEM) 2022 report. Multidimensional assessment is desirable and might be best based on measurement of global functional impairment. More work is required to develop and implement recommendations for multidimensional assessment. Prediction of outcome is relevant to patients and their families, and can facilitate the benchmarking of quality of care. InTBIR studies have identified new building blocks (eg, blood biomarkers and quantitative CT analysis) to refine existing prognostic models. Further improvement in prognostication could come from MRI, genetics, and the integration of dynamic changes in patient status after presentation.
Neurotrauma researchers traditionally seek translation of their research findings through publications, clinical guidelines, and industry collaborations. However, to effectively impact clinical care and outcome, interactions are also needed with research funders, regulators, and policy makers, and partnership with patient organisations. Such interactions are increasingly taking place, with exemplars including interactions with the All Party Parliamentary Group on Acquired Brain Injury in the UK, the production of the NASEM report in the USA, and interactions with the US Food and Drug Administration. More interactions should be encouraged, and future discussions with regulators should include debates around consent from patients with acute mental incapacity and data sharing. Data sharing is strongly advocated by funding agencies. From January 2023, the US National Institutes of Health will require upload of research data into public repositories, but the EU requires data controllers to safeguard data security and privacy regulation. The tension between open data-sharing and adherence to privacy regulation could be resolved by cross-dataset analyses on federated platforms, with the data remaining at their original safe location. Tools already exist for conventional statistical analyses on federated platforms, however federated machine learning requires further development. Support for further development of federated platforms, and neuroinformatics more generally, should be a priority.
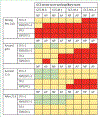
This update to the 2017 Commission presents new insights and challenges across a range of topics around TBI: epidemiology and prevention (section 1); system of care (section 2); clinical management (section 3); characterisation of TBI (section 4); outcome assessment (section 5); prognosis (Section 6); and new directions for acquiring and implementing evidence (section 7). Table 1 summarises key messages from this Commission and proposes recommendations for the way forward to advance research and clinical management of TBI.
Conflict of interest statement
Declaration of interests No funding was provided specifically for this Commission paper; however, most authors are involved in the International Initiative for Traumatic Brain Injury Research (InTBIR) as a scientific participant or an investigator. This Commission would not have been possible without the indirect facilitation provided by the InTBIR network. AIRM declares consulting fees from PresSura Neuro, Integra Life Sciences, and NeuroTrauma Sciences. DKM reports research support, and educational and consulting fees from Lantmannen AB, GlaxoSmithKline, Calico, PresSura Neuro, NeuroTrauma Sciences, and Integra Neurosciences. GTM declares grants from the US National Institutes of Health-National Institute of Neurological Disorders and Stroke (grant U01NS086090), the US Department of Defense (grant W81XWH-14-2-0176, grant W81XWH-18-2-0042, and contract W81XWH-15-9-0001). MC reports licensing fees for ICM+ software from Cambridge Enterprise and was an honorary (unpaid) director for Medicam. PS reports licensing fees for ICM+ software from Cambridge Enterprise. MBS has in the past 3 years received consulting income from Acadia Pharmaceuticals, Aptinyx, atai Life Sciences, Boehringer Ingelheim, Bionomics, BioXcel Therapeutics, Clexio, Eisai, EmpowerPharm, Engrail Therapeutics, Janssen, Jazz Pharmaceuticals, and Roche/Genentech. MBS also has stock options in Oxeia Biopharmaceuticals and EpiVario and is paid for editorial work on Depression and Anxiety (Editor-in-Chief), Biological Psychiatry (Deputy Editor), and UpToDate (Co-Editor-in-Chief for Psychiatry). KKWW holds stock options in Gryphon Bio. All other authors declare no competing interests.
Figures




Comment in
-
Towards further progress in traumatic brain injury.Lancet Neurol. 2023 Feb;22(2):109. doi: 10.1016/S1474-4422(22)00528-2. Lancet Neurol. 2023. PMID: 36681439 No abstract available.
References
-
- Maas AIR, Menon DK, Adelson PD, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017; 16: 987–1048. - PubMed
-
- Menon DK, Bryant C. Time for change in acquired brain injury. Lancet Neurol 2019; 18: 28. - PubMed
-
- Majdan M, Plancikova D, Brazinova A, et al. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health 2016; 1: e76–83. - PubMed
-
- Bell MJ, Kochanek PM. International traumatic brain injury research: an annus mirabilis? Lancet Neurol 2019; 18: 904–05. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

