Presence of Intact Hepatitis B Virions in Exosomes
- PMID: 36184032
- PMCID: PMC9676402
- DOI: 10.1016/j.jcmgh.2022.09.012
Presence of Intact Hepatitis B Virions in Exosomes
Abstract
Background & aims: Hepatitis B virus (HBV) was identified as an enveloped DNA virus with a diameter of 42 nm. Multivesicular bodies play a central role in HBV egress and exosome biogenesis. In light of this, it was studied whether intact virions wrapped in exosomes are released by HBV-producing cells.
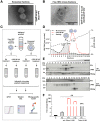
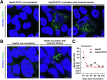
Methods: Robust methods for efficient separation of exosomes from virions were established. Exosomes were subjected to limited detergent treatment for release of viral particles. Electron microscopy of immunogold labeled ultrathin sections of purified exosomes was performed for characterization of exosomal HBV. Exosome formation/release was affected by inhibitors or Crispr/Cas-mediated gene silencing. Infectivity/uptake of exosomal HBV was investigated in susceptible and non-susceptible cells.
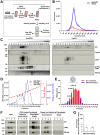
Results: Exosomes could be isolated from supernatants of HBV-producing cells, which are characterized by the presence of exosomal and HBV markers. These exosomal fractions could be separated from the fractions containing free virions. Limited detergent treatment of exosomes causes stepwise release of intact HBV virions and naked capsids. Inhibition of exosome morphogenesis impairs the release of exosome-wrapped HBV. Electron microscopy confirmed the presence of intact virions in exosomes. Moreover, the presence of large hepatitis B virus surface antigen on the surface of exosomes derived from HBV expressing cells was observed, which conferred exosome-encapsulated HBV initiating infection in susceptible cells in a , large hepatitis B virus surface antigen/Na+-taurocholate co-transporting polypeptide-dependent manner. The uptake of exosomal HBV with low efficiency was also observed in non-permissive cells.
Conclusion: These data indicate that a fraction of intact HBV virions can be released as exosomes. This reveals a so far not described release pathway for HBV.
Keywords: MVB; Ultrathin Cryosection; Virion Egress; Virus-host Interaction.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Glebe D., König A. Molecular virology of hepatitis B virus and targets for antiviral intervention. Intervirology. 2014;57:134–140. - PubMed
-
- Sun D., Nassal M. Stable HepG2- and Huh7-based human hepatoma cell lines for efficient regulated expression of infectious hepatitis B virus. J Hepatol. 2006;45:636–645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

