Estimating vaccine effectiveness against SARS-CoV-2 infection, hospitalization and death from ecologic data in Costa Rica
- PMID: 36184587
- PMCID: PMC9526815
- DOI: 10.1186/s12879-022-07740-5
Estimating vaccine effectiveness against SARS-CoV-2 infection, hospitalization and death from ecologic data in Costa Rica
Abstract
Background: Clinical trials and individual-level observational data in Israel demonstrated approximately 95% effectiveness of mRNA-based vaccines against symptomatic SARS-CoV-2 infection. Individual-level data are not available in many countries, particularly low- and middle- income countries. Using a novel Poisson regression model, we analyzed ecologic data in Costa Rica to estimate vaccine effectiveness and assess the usefulness of this approach.
Methods: We used national data from December 1, 2020 to May 13, 2021 to ascertain incidence, hospitalizations and deaths within ecologic units defined by 14 age groups, gender, 105 geographic areas, and day of the epidemic. Within each unit we used the proportions of the population with one and with two vaccinations, primarily tozinameran. Using a non-standard Poisson regression model that included an ecologic-unit-specific rate factor to describe rates without vaccination and a factor that depended on vaccine effectiveness parameters and proportions vaccinated, we estimated vaccine effectiveness.
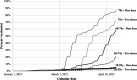
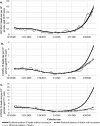
Results: In 3.621 million persons aged 20 or older, there were 125,031 incident cases, 7716 hospitalizations, and 1929 deaths following SARS-CoV-2 diagnosis; 73% of those aged ≥ 75 years received two doses. For one dose, estimated effectiveness was 59% (95% confidence interval 53% to 64%) for SARS-CoV-2 incidence, 76% (68% to 85%) for hospitalizations, and 63% (47% to 80%) for deaths. For two doses, the respective estimates of effectiveness were 93% (90% to 96%), 100% (97% to 100%), and 100% (97% to 100%).
Conclusions: These effectiveness estimates agree well with findings from clinical trials and individual-level observational studies and indicate high effectiveness in the general population of Costa Rica. This novel statistical approach is promising for countries where ecologic, but not individual-level, data are available. The method could also be adapted to monitor vaccine effectiveness over calendar time.
Keywords: COVID-19; Ecologic estimate of vaccine effectiveness; SARS-CoV-2; Vaccine effectiveness.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan KJ, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. - DOI - PMC - PubMed
-
- Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, AlMukdad S, Coyle P, Ayoub HH, Al Kanaani Z, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27(12):2136–2143. doi: 10.1038/s41591-021-01583-4. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

