The alarmin interleukin-1α triggers secondary degeneration through reactive astrocytes and endothelium after spinal cord injury
- PMID: 36184639
- PMCID: PMC9527244
- DOI: 10.1038/s41467-022-33463-x
The alarmin interleukin-1α triggers secondary degeneration through reactive astrocytes and endothelium after spinal cord injury
Abstract
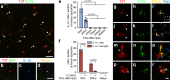
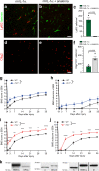
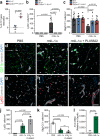
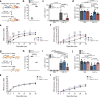
Spinal cord injury (SCI) triggers neuroinflammation, and subsequently secondary degeneration and oligodendrocyte (OL) death. We report that the alarmin interleukin (IL)-1α is produced by damaged microglia after SCI. Intra-cisterna magna injection of IL-1α in mice rapidly induces neutrophil infiltration and OL death throughout the spinal cord, mimicking the injury cascade seen in SCI sites. These effects are abolished through co-treatment with the IL-1R1 antagonist anakinra, as well as in IL-1R1-knockout mice which demonstrate enhanced locomotor recovery after SCI. Conditional restoration of IL-1R1 expression in astrocytes or endothelial cells (ECs), but not in OLs or microglia, restores IL-1α-induced effects, while astrocyte- or EC-specific Il1r1 deletion reduces OL loss. Conditioned medium derived from IL-1α-stimulated astrocytes results in toxicity for OLs; further, IL-1α-stimulated astrocytes generate reactive oxygen species (ROS), and blocking ROS production in IL-1α-treated or SCI mice prevented OL loss. Thus, after SCI, microglia release IL-1α, inducing astrocyte- and EC-mediated OL degeneration.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- David, S. & Lacroix, S. in Clinical Neuroimmunology (eds J. Antel, G. Birnbaum, H. P. Hartung, & A. Vincent) 53-63 (Oxford University press, 2005).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

