WNT-modulating gene silencers as a gene therapy for osteoporosis, bone fracture, and critical-sized bone defects
- PMID: 36184851
- PMCID: PMC9931550
- DOI: 10.1016/j.ymthe.2022.09.018
WNT-modulating gene silencers as a gene therapy for osteoporosis, bone fracture, and critical-sized bone defects
Abstract
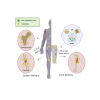
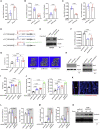
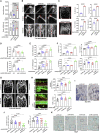
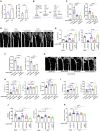
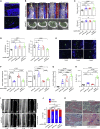
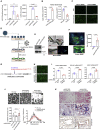
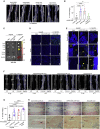
Treating osteoporosis and associated bone fractures remains challenging for drug development in part due to potential off-target side effects and the requirement for long-term treatment. Here, we identify recombinant adeno-associated virus (rAAV)-mediated gene therapy as a complementary approach to existing osteoporosis therapies, offering long-lasting targeting of multiple targets and/or previously undruggable intracellular non-enzymatic targets. Treatment with a bone-targeted rAAV carrying artificial microRNAs (miRNAs) silenced the expression of WNT antagonists, schnurri-3 (SHN3), and sclerostin (SOST), and enhanced WNT/β-catenin signaling, osteoblast function, and bone formation. A single systemic administration of rAAVs effectively reversed bone loss in both postmenopausal and senile osteoporosis. Moreover, the healing of bone fracture and critical-sized bone defects was also markedly improved by systemic injection or transplantation of AAV-bound allograft bone to the osteotomy sites. Collectively, our data demonstrate the clinical potential of bone-specific gene silencers to treat skeletal disorders of low bone mass and impaired fracture repair.
Keywords: bone fracture; critical-sized bone defect; osteoblast; osteoclast; osteoporosis; rAAV; schnurri-3; sclerostin; skeletal organoid.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.-H.S. is a scientific co-founder of AAVAA Therapeutics and holds equity in this company. G.G. is a scientific co-founder of AAVAA Therapeutics, Voyager Therapeutics, and Aspa Therapeutics and holds equity in these companies. G.G. is an inventor on patents with potential royalties licensed to Voyager Therapeutics, Aspa Therapeutics, and other biopharmaceutical companies. D.S.O. is a chief scientific officer of Osteogene Tech. These pose no conflicts for this study. The other authors declare no competing interests.
Figures
References
-
- Eastell R., Christiansen C., Grauer A., Kutilek S., Libanati C., McClung M.R., et al. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J. Bone Miner Res. 2011;26:530–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

