Surface-Enriched Boron-Doped TiO2 Nanoparticles as Photocatalysts for Propene Oxidation
- PMID: 36185169
- PMCID: PMC9513816
- DOI: 10.1021/acsanm.2c02217
Surface-Enriched Boron-Doped TiO2 Nanoparticles as Photocatalysts for Propene Oxidation
Abstract
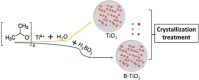
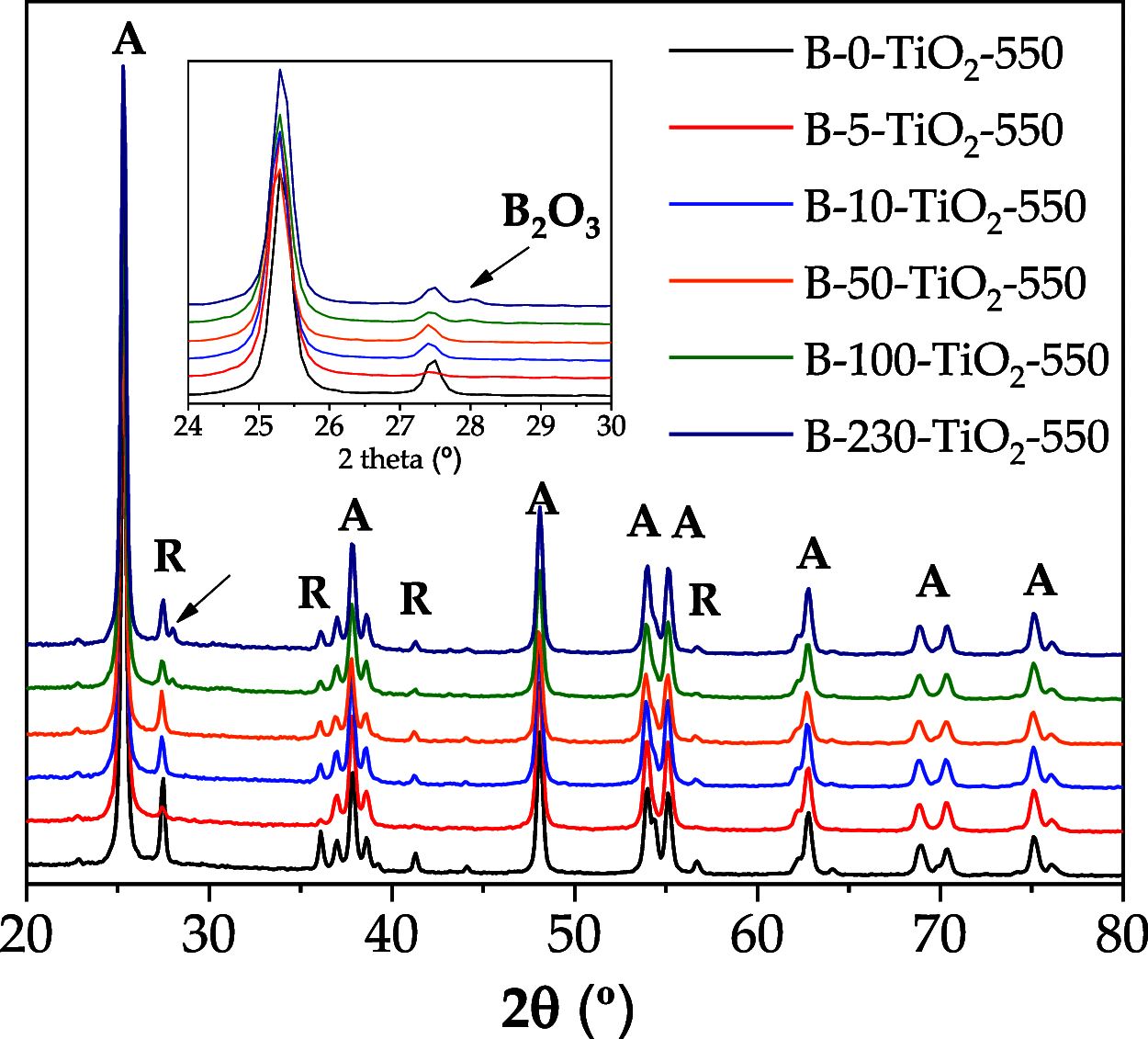
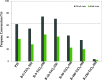
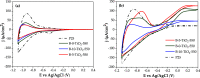
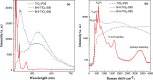
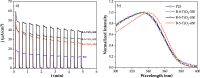
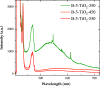
A series of nanostructured boron-TiO2 photocatalysts (B-X-TiO2-T) were prepared by sol-gel synthesis using titanium tetraisopropoxide and boric acid. The effects of the synthesis variables, boric acid amount (X) and crystallization temperature (T), on structural and electronic properties and on the photocatalytic performance for propene oxidation, are studied. This reaction accounts for the remediation of pollution caused by volatile organic compounds, and it is carried out at low concentrations, a case in which efficient removal techniques are difficult and costly to implement. The presence of boric acid during the TiO2 synthesis hinders the development of rutile without affecting the textural properties. X-ray photoelectron spectroscopy analysis reveals the interstitial incorporation of boron into the surface lattice of the TiO2 nanostructure, while segregation of B2O3 occurs in samples with high boron loading, also confirmed by X-ray diffraction. The best-performing photocatalysts are those with the lowest boron loading. Their high activity, outperforming the equivalent sample without boron, can be attributed to a high anatase and surface hydroxyl group content and efficient photo-charge separation (photoelectrochemical characterization, PEC), which can explain the suppression of visible photoluminescence (PL). Crystallization at 450 °C renders the most active sample, likely due to the development of a pure anatase structure with a large surface boron enrichment. A shift in the wavelength-dependent activity profile (PEC data) and the lowest electron-hole recombination rate (PL data) are also observed for this sample.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Soni V.; Singh P.; Shree V.; Goel V.. Effects of VOCs on Human Health; Springer, 2018; pp 119–142.
-
- Lillo-Ródenas M. A.; Bouazza N.; Berenguer-Murcia A.; Linares-Salinas J.; Soto P.; Linares-Solano A. Photocatalytic Oxidation of Propene at Low Concentration. Appl. Catal., B 2007, 71 (3-4), 298–309. 10.1016/j.apcatb.2006.10.004. - DOI
-
- Ouzzine M.; Lillo-Ródenas M. A.; Linares-Solano A. Photocatalytic Oxidation of Propene in Gas Phase at Low Concentration by Optimized TiO2 Nanoparticles. Appl. Catal., B 2013, 134-135, 333–343. 10.1016/j.apcatb.2013.01.035. - DOI
-
- Ghoshal A. K.; Manjare S. D. Selection of Appropriate Adsorption Technique for Recovery of VOCs: An Analysis. J. Loss Prev. Process Ind. 2002, 15, 413–421. 10.1016/S0950-4230(02)00042-6. - DOI
-
- Alberici R. M.; Jardim W. F.. Photocatalytic Destruction of VOCS in the Gas-Phase Using Titanium Dioxide.Appl. Catal., B 1997, 14 (), 55–68. https://doi.org/10.1016/S0926-3373(97)00012-X. - DOI
LinkOut - more resources
Full Text Sources
