Comparative Adherence and Persistence of Single- and Multiple-Inhaler Triple Therapies Among Patients with Chronic Obstructive Pulmonary Disease in an English Real-World Primary Care Setting
- PMID: 36185170
- PMCID: PMC9519012
- DOI: 10.2147/COPD.S370540
Comparative Adherence and Persistence of Single- and Multiple-Inhaler Triple Therapies Among Patients with Chronic Obstructive Pulmonary Disease in an English Real-World Primary Care Setting
Abstract
Purpose: Triple therapy comprising a long-acting muscarinic antagonist, long-acting β2-agonist and inhaled corticosteroid is recommended for patients with chronic obstructive pulmonary disease (COPD) who continue to experience frequent exacerbations or symptoms whilst receiving dual therapy. Adherence and persistence to multiple-inhaler triple therapy (MITT) is known to be poor. This study assessed comparative adherence to single-inhaler triple therapy (SITT) versus MITT in a real-world setting in England.
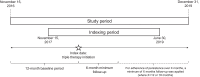
Patients and methods: This was a retrospective cohort study using linked primary care (Clinical Practice Research Datalink Aurum) and secondary care (Hospital Episode Statistics [HES] Admitted Patient Care) data to identify patients with COPD who were newly initiated on SITT or MITT between November 2017 and June 2019. Eligible patients were aged ≥35 years and had a forced expiratory volume in 1 second/forced vital capacity <0.7, linkage to HES and continuous registration with a general practitioner for 12 months pre- and 6 months post-initiation. Inverse probability of treatment weighting was used to balance baseline characteristics between cohorts. Adherence was measured using the proportion of days covered by days' supply of SITT or MITT prescriptions. Persistence was measured with a gap of >30 days between the end of a prescription and the following refill used to determine non-persistence.
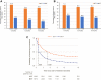
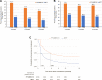
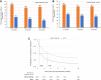
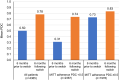
Results: Overall, 4080 SITT and 6579 MITT users comprised the study cohort. After weighting, the baseline characteristics between the cohorts were comparable (absolute standardized mean difference <10%). SITT users had significantly higher adherence than MITT users at 6, 12, and 18 months post-initiation (p<0.001 for all comparisons). Median persistence was higher among SITT users than MITT users (5.09 months vs 0.99 months).
Conclusion: Patients with COPD in England initiating SITT had significantly better adherence and persistence compared with MITT initiators. These improvements continued at least 18 months following treatment initiation.
Keywords: COPD; Clinical Practice Research Datalink; Hospital Episode Statistics; MITT; SITT; proportion of days covered.
© 2022 Halpin et al.
Conflict of interest statement
Kieran J Rothnie, Alexandrosz Czira, Chris Compton, Kirill Nikitin, Raj Sharma, and Afisi S Ismaila are employees of and/or hold stocks/shares in GSK. Neil Snowise is a previous employee of GSK and holds GSK stocks/shares. Victoria Banks, Robert Wood, Theo Tritton, Olivia Massey, and Rosie Wild are employees of Adelphi Real World. Adelphi Real World received funding from GSK to conduct the study. David MG Halpin reports personal fees from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, personal fees from Aerogen, Chiesi, CSL Behring, and GSK, personal fees and non-financial support from Novartis, and personal fees from Pfizer and Sanofi. Claus F Vogelmeier has received research grants from AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GSK, and Grifols, and has given presentations at symposia and/or served on scientific advisory boards sponsored by Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GSK, Grifols, Menarini, Novartis, and Nuvaira. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Evaluation of Adherence and Persistence to Triple Therapy in Patients with COPD: A German Claims Data Study.Int J Chron Obstruct Pulmon Dis. 2024 Aug 9;19:1835-1848. doi: 10.2147/COPD.S460903. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 39140078 Free PMC article.
-
Characteristics of Users and New Initiators of Single- and Multiple-Inhaler Triple Therapy for Chronic Obstructive Pulmonary Disease in Germany.Int J Chron Obstruct Pulmon Dis. 2024 Apr 17;19:945-956. doi: 10.2147/COPD.S431291. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 38646606 Free PMC article.
-
Clinical Characteristics, Treatment Persistence, and Outcomes Among Patients With COPD Treated With Single- or Multiple-Inhaler Triple Therapy: A Retrospective Analysis in Spain.Chest. 2022 Nov;162(5):1017-1029. doi: 10.1016/j.chest.2022.06.033. Epub 2022 Jul 3. Chest. 2022. PMID: 35787391
-
Adherence to single inhaler triple therapy and digital inhalers in Chronic Obstructive Pulmonary Disease: a literature review and protocol for a randomized controlled trial (TRICOLON study).BMC Pulm Med. 2024 Jul 4;24(1):317. doi: 10.1186/s12890-024-03044-3. BMC Pulm Med. 2024. PMID: 38965541 Free PMC article. Review.
-
Clinical characteristics, treatment patterns and adherence in patients with asthma on multiple inhaler triple therapy: a review of findings.Expert Rev Respir Med. 2022 Nov-Dec;16(11-12):1205-1212. doi: 10.1080/17476348.2023.2167715. Epub 2023 Jan 17. Expert Rev Respir Med. 2022. PMID: 36629483 Review.
Cited by
-
Single inhaler with beclometasone, formoterol, and glycopyrronium versus triple therapies in adults with uncontrolled asthma: a systematic review and meta-analysis.Sci Rep. 2025 Feb 4;15(1):4191. doi: 10.1038/s41598-025-88374-w. Sci Rep. 2025. PMID: 39905183 Free PMC article.
-
The Real-World Efficacy of Fixed Triple Inhalation Therapy in the Treatment of Moderate COPD Patients (RATIONALE Study).Int J Chron Obstruct Pulmon Dis. 2024 Aug 28;19:1943-1955. doi: 10.2147/COPD.S474354. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 39219564 Free PMC article.
-
Peak Inspiratory Flow and Inhaler Prescription Strategies in a Specialized COPD Clinical Program: A Real-World Observational Study.Chest. 2025 Mar;167(3):736-745. doi: 10.1016/j.chest.2024.09.031. Epub 2024 Oct 9. Chest. 2025. PMID: 39384100 Free PMC article.
-
Characteristics of Patients Receiving Budesonide/Glycopyrronium/Formoterol for Chronic Obstructive Pulmonary Disease in Spain: The AURA Study.Int J Chron Obstruct Pulmon Dis. 2025 Apr 9;20:999-1008. doi: 10.2147/COPD.S490227. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 40226230 Free PMC article.
-
Real-World Effectiveness of Single-Inhaler Triple Treatment Through Assorted Respiratory Outcomes When Switched From Multiple-Inhaler Triple Therapies (RESTART): A Prospective Cohort Study of Korean Patients With COPD.Int J Chron Obstruct Pulmon Dis. 2025 Apr 11;20:1039-1050. doi: 10.2147/COPD.S499686. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 40236761 Free PMC article.
References
-
- GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease - 2021 report; 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25.... Accessed January 17, 2022.
-
- Bogart M, Stanford RH, Laliberté F, Germain G, Wu JW, Duh MS. Medication adherence and persistence in chronic obstructive pulmonary disease patients receiving triple therapy in a USA commercially insured population. Int J Chron Obstruct Pulmon Dis. 2019;14:343. doi:10.2147/COPD.S184653 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

