Myostatin/HIF2α-Mediated Ferroptosis is Involved in Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease
- PMID: 36185172
- PMCID: PMC9519128
- DOI: 10.2147/COPD.S377226
Myostatin/HIF2α-Mediated Ferroptosis is Involved in Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease
Abstract
Objective: Skeletal muscle dysfunction is an important comorbidity in patients with chronic obstructive pulmonary disease (COPD), and is associated with poor quality of life and reduced survival, but the mechanisms involved remain elusive. Ferroptosis is a newly discovered type of cell death resulting from iron-dependent lipid peroxide accumulation. The purpose of this study was to examine whether ferroptosis is involved in COPD-associated skeletal muscle dysfunction.
Methods: A mouse model of COPD was established after 24 weeks of cigarette smoke (CS) exposure, and mRNA sequencing, hematoxylin-eosin (H&E) staining, immunostaining (IF), RT-PCR, and Western blot were utilized to identify the changes in gastrocnemius muscles. In vitro, C2C12 myotubes were treated with CS extract (CSE) and evaluated for ferroptosis-related molecules. The pathways regulating ferroptosis were then explored in CSE-stimulated myotubes.
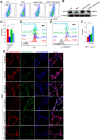
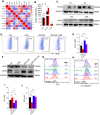
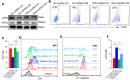
Results: Compared with controls, COPD mice showed an enriched ferroptosis pathway. Gpx4 was decreased, while hypoxia-inducible factor (Hif) 2α was increased, at gene and protein levels. A reduced level of GSH, but increased cell death, Fe2+, lipid ROS, LPO, and 4-HNE were observed in COPD mice or in CSE-stimulated C2C12 myotubes, which could be ameliorated by ferroptosis inhibitors. The expression of myostatin (MSTN) was enhanced in COPD mice and CSE-stimulated myotubes. MSTN up-regulated HIF2α expression and led to ferroptosis in myotubes, whereas inhibition of MSTN binding to its receptor or inhibition/knockdown of HIF2α resulted in decreased cell death, and partially restored GPX4 and GSH.
Conclusion: CS exposure induced ferroptosis in vivo and in vitro. Mechanistically, CS-exposure upregulated MSTN which further induced ferroptosis through HIF2α in skeletal muscles, which may contribute to muscle dysfunction through impairing metabolic capacity and decreasing muscle fiber numbers, revealing a potential novel therapeutic target for COPD-related skeletal muscle dysfunction.
Keywords: chronic obstructive pulmonary disease; ferroptosis; hypoxia-inducible factor 2α; myostatin; skeletal muscle dysfunction.
© 2022 Zhang et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
RANKL Mediates Muscle Atrophy and Dysfunction in a Cigarette Smoke-induced Model of Chronic Obstructive Pulmonary Disease.Am J Respir Cell Mol Biol. 2021 May;64(5):617-628. doi: 10.1165/rcmb.2020-0449OC. Am J Respir Cell Mol Biol. 2021. PMID: 33689672
-
Dysregulated myokines and signaling pathways in skeletal muscle dysfunction in a cigarette smoke-induced model of chronic obstructive pulmonary disease.Front Physiol. 2022 Aug 25;13:929926. doi: 10.3389/fphys.2022.929926. eCollection 2022. Front Physiol. 2022. PMID: 36091368 Free PMC article.
-
Dihydroquercetin suppresses cigarette smoke induced ferroptosis in the pathogenesis of chronic obstructive pulmonary disease by activating Nrf2-mediated pathway.Phytomedicine. 2022 Feb;96:153894. doi: 10.1016/j.phymed.2021.153894. Epub 2021 Dec 14. Phytomedicine. 2022. PMID: 34942457
-
Ferroptosis and Its Potential Role in the Physiopathology of Skeletal Muscle Atrophy.Int J Mol Sci. 2024 Nov 20;25(22):12463. doi: 10.3390/ijms252212463. Int J Mol Sci. 2024. PMID: 39596528 Free PMC article. Review.
-
Autophagy in skeletal muscle dysfunction of chronic obstructive pulmonary disease: implications, mechanisms, and perspectives.J Zhejiang Univ Sci B. 2025 Mar 1;26(3):227-239. doi: 10.1631/jzus.B2300680. J Zhejiang Univ Sci B. 2025. PMID: 40082202 Free PMC article. Review.
Cited by
-
The Importance of the Diaphragm in Neuromotor Function in the Patient with Chronic Obstructive Pulmonary Disease.Int J Chron Obstruct Pulmon Dis. 2023 May 11;18:837-848. doi: 10.2147/COPD.S404190. eCollection 2023. Int J Chron Obstruct Pulmon Dis. 2023. PMID: 37197600 Free PMC article. Review.
-
Main Pathogenic Mechanisms and Recent Advances in COPD Peripheral Skeletal Muscle Wasting.Int J Mol Sci. 2023 Mar 29;24(7):6454. doi: 10.3390/ijms24076454. Int J Mol Sci. 2023. PMID: 37047427 Free PMC article. Review.
-
Exerkines: Potential regulators of ferroptosis.J Sport Health Sci. 2025 Feb 21;14:101032. doi: 10.1016/j.jshs.2025.101032. Online ahead of print. J Sport Health Sci. 2025. PMID: 39988270 Free PMC article. Review.
-
YAP/TAZ-mediated nuclear membrane rupture in promoting senescence of skeletal muscle associated with COPD.Respir Res. 2025 Mar 12;26(1):98. doi: 10.1186/s12931-025-03170-4. Respir Res. 2025. PMID: 40075503 Free PMC article.
-
Myostatin: a potential therapeutic target for metabolic syndrome.Front Endocrinol (Lausanne). 2023 May 23;14:1181913. doi: 10.3389/fendo.2023.1181913. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37288303 Free PMC article. Review.
References
-
- Vogelmeier CAA, Anzueto A, Barnes P, Bourbeau J, Criner G. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. In: 2021 Report. Global Initiat Chronic Obstruct Lung Dis. http://www.goldcopd.org.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

