Comprehensive multi-omics single-cell data integration reveals greater heterogeneity in the human immune system
- PMID: 36185375
- PMCID: PMC9523353
- DOI: 10.1016/j.isci.2022.105123
Comprehensive multi-omics single-cell data integration reveals greater heterogeneity in the human immune system
Abstract
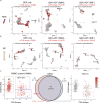
Single-cell transcriptomics enables the definition of diverse human immune cell types across multiple tissues and disease contexts. Further deeper biological understanding requires comprehensive integration of multiple single-cell omics (transcriptomic, proteomic, and cell-receptor repertoire). To improve the identification of diverse cell types and the accuracy of cell-type classification in multi-omics single-cell datasets, we developed SuPERR, a novel analysis workflow to increase the resolution and accuracy of clustering and allow for the discovery of previously hidden cell subsets. In addition, SuPERR accurately removes cell doublets and prevents widespread cell-type misclassification by incorporating information from cell-surface proteins and immunoglobulin transcript counts. This approach uniquely improves the identification of heterogeneous cell types and states in the human immune system, including rare subsets of antibody-secreting cells in the bone marrow.
Keywords: Biocomputational method; Omics; Systems biology.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Altman N., Krzywinski M. The curse(s) of dimensionality. Nat. Methods. 2018;15:399–400. - PubMed
-
- Babcock B.R., Kosters A., Yang J., White L., Ghosn E.B. Data matrix normalization and merging strategies minimize batch-specific systemic variation in scRNA-seq data. bioRxiv. 2021 doi: 10.1101/2021.08.18.456898. Preprint at. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

