World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) guideline update - XIII - Oral immunotherapy for CMA - Systematic review
- PMID: 36185550
- PMCID: PMC9474924
- DOI: 10.1016/j.waojou.2022.100682
World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) guideline update - XIII - Oral immunotherapy for CMA - Systematic review
Abstract
Background: Allergy to cow's milk is the most common food allergy in infants and it is usually outgrown by 5 years of age. In some individuals it persists beyond early childhood. Oral immunotherapy (OIT, oral desensitization, specific oral tolerance induction) has been proposed as a promising therapeutic strategy for persistent IgE-mediated cow's milk allergy. We previously published the systematic review of OIT for cow's milk allergy (CMA) in 2010 as part of the World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines.
Objective: To systematically synthesize the currently available evidence about OIT for IgE-mediated CMA and to inform the updated 2022 WAO guidelines.
Methods: We searched the electronic databases including PubMed, Medline, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and the websites of selected allergy organizations. We included all studies irrespective of the language of the original publication. The last search was conducted in February 2021. We registered the protocol on Open Science Framework (10.17605/OSF.IO/AH2DT).
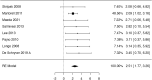
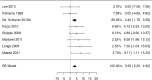
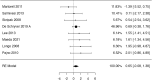
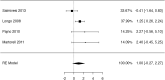
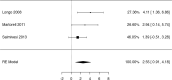
Results: We identified 2147 unique records published between 2010 and 2021, including 13 randomized trials and 109 observational studies addressing cow's milk OIT. We found low-certainty evidence that OIT with unheated cow's milk, compared to elimination diet alone, increased the likelihood of being able to consume ≥150 ml of cow's milk in controlled settings (risk ratio (RR): 12.3, 95% CI: 5.9 to 26.0; risk difference (RD): 25 more per 100, 95% CI 11 to 56) as well as accidently ingest a small amount (≥5 ml) of cow's milk (RR: 8.7, 95% CI: 4.7 to 16.1; RD: 25 more per 100, 95% CI 12 to 50). However, 2-8 weeks after discontinuation of a successful OIT, tolerance of cow's milk persisted in only 36% (range: 20%-91%) of patients. OIT increased the frequency of anaphylaxis (rate ratio: 60.0, 95% CI 15 to 244; rate difference 5 more anaphylactic reactions per 1 person per year, 95% CI: 4 to 6; moderate evidence) and the frequency of epinephrine use (rate ratio: 35.2, 95% CI: 9 to 136.5; rate difference 268 more events per 100 person-years, 95% CI: 203 to 333; high certainty). OIT also increased the risk of gastrointestinal symptoms (RR 6.9, 95% CI 1.6-30.9; RD 28 more per 100, CI 3 to 100) and respiratory symptoms (RR 49.0, 95% CI 3.12-770.6; RD 77 more per 100, CI 62 to 92), compared with avoidance diet alone. Single-arm observational studies showed that on average 6.9% of OIT patients (95% CI: 3.8%-10%) developed eosinophilic esophagitis (very low certainty evidence). We found 1 trial and 2 small case series of OIT with baked milk.
Conclusions: Moderate certainty evidence shows that OIT with unheated cow's milk in patients with IgE-mediated CMA is associated with an increased probability of being able to drink milk and, at the same time, an increased risk of serious adverse effects.
Keywords: GRADE; Meta-analysis; Milk allergy; Oral immunotherapy; Systematic review.
© 2022 Published by Elsevier Inc. on behalf of World Allergy Organization.
Figures




References
-
- Rona R.J., Keil T., Summers C., et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–646. - PubMed
-
- Nwaru B.I., Hickstein L., Panesar S.S., et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014;69(1):62–75. - PubMed
-
- Schoemaker A.A., Sprikkelman A.B., Grimshaw K.E., et al. Incidence and natural history of challenge-proven cow's milk allergy in European children–EuroPrevall birth cohort. Allergy. 2015;70(8):963–972. - PubMed
-
- Spergel J.M. Natural history of cow's milk allergy. J Allergy Clin Immunol. 2013;131(3):813–814. - PubMed
-
- Hill D.J., Firer M.A., Ball G., Hosking C.S. Natural history of cows' milk allergy in children: immunological outcome over 2 years. Clin Exp Allergy. 1993;23(2):124–131. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

