Comprehensive overview of Nrf2-related epigenetic regulations involved in ischemia-reperfusion injury
- PMID: 36185600
- PMCID: PMC9516229
- DOI: 10.7150/thno.77243
Comprehensive overview of Nrf2-related epigenetic regulations involved in ischemia-reperfusion injury
Abstract
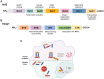
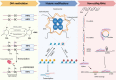
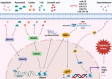
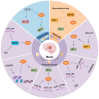
Ischemic disease is a class of diseases in which an organ is ischemic due to vascular occlusion, a major contributor to death and disability worldwide. However, when the blood flow is restored, more severe damage occurs than ischemia alone and is known as ischemic-reperfusion injury (IRI). During reperfusion, the imbalance between the production of reactive oxygen species (ROS) and buffering capacity of the antioxidant defense system results in cell damage and death. Nuclear factor E2-related factor 2 (Nrf2) significantly affects antioxidant stress damage. The function of Nrf2 in the pathological process of IRI has been widely discussed, but the impact of epigenetic modifications associated with Nrf2 remains unclear. This article provides a comprehensive overview of the role and mechanism of Nrf2-related epigenetic modifications in the IRI of various organs, including the brain, heart, liver, and kidney. In addition, we summarize agonists that may target epigenetic regulation of Nrf2, which may be beneficial in seeking more effective strategies to improve IRI.
Keywords: Nrf2; epigenetic modifications; ischemic-reperfusion injury; oxidative stress; therapy.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS. et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. - PubMed
-
- Doenst T, Haverich A, Serruys P, Bonow RO, Kappetein P, Falk V. et al. PCI and CABG for Treating Stable Coronary Artery Disease: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73:964–76. - PubMed
-
- McCarthy CP, Vaduganathan M, McCarthy KJ, Januzzi JL Jr, Bhatt DL, McEvoy JW. Left Ventricular Thrombus After Acute Myocardial Infarction: Screening, Prevention, and Treatment. JAMA Cardiol. 2018;3:642–9. - PubMed
-
- Khan H, Gupta A, Singh TG, Kaur A. Mechanistic insight on the role of leukotriene receptors in ischemic-reperfusion injury. Pharmacol Rep. 2021;73:1240–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

