Liver-specific drug delivery platforms: Applications for the treatment of alcohol-associated liver disease
- PMID: 36185629
- PMCID: PMC9521517
- DOI: 10.3748/wjg.v28.i36.5280
Liver-specific drug delivery platforms: Applications for the treatment of alcohol-associated liver disease
Abstract
Alcohol-associated liver disease (ALD) is a common chronic liver disease and major contributor to liver disease-related deaths worldwide. Despite its pre-valence, there are few effective pharmacological options for the severe stages of this disease. While much pre-clinical research attention is paid to drug development in ALD, many of these experimental therapeutics have limitations such as poor pharmacokinetics, poor efficacy, or off-target side effects due to systemic administration. One means of addressing these limitations is through liver-targeted drug delivery, which can be accomplished with different platforms including liposomes, polymeric nanoparticles, exosomes, bacteria, and adeno-associated viruses, among others. These platforms allow drugs to target the liver passively or actively, thereby reducing systemic circulation and increasing the 'effective dose' in the liver. While many studies, some clinical, have applied targeted delivery systems to other liver diseases such as viral hepatitis or hepatocellular carcinoma, only few have investigated their efficacy in ALD. This review provides basic information on these liver-targeting drug delivery platforms, including their benefits and limitations, and summarizes the current research efforts to apply them to the treatment of ALD in rodent models. We also discuss gaps in knowledge in the field, which when addressed, may help to increase the efficacy of novel therapies and better translate them to humans.
Keywords: Alcohol associated liver disease; Liposomes; Liver targeted delivery; Nanoparticles; Polymeric nanoparticles; Precision medicine.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures

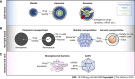

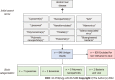
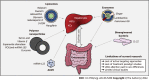
Similar articles
-
New molecular targets for functionalized nanosized drug delivery systems in personalized therapy for hepatocellular carcinoma.J Control Release. 2017 Dec 28;268:184-197. doi: 10.1016/j.jconrel.2017.10.027. Epub 2017 Oct 16. J Control Release. 2017. PMID: 29051062 Review.
-
Alcoholic-related liver disease: pathogenesis, management and future therapeutic developments.Rev Esp Enferm Dig. 2020 Nov;112(11):869-878. doi: 10.17235/reed.2020.7242/2020. Rev Esp Enferm Dig. 2020. PMID: 33054302
-
Lipid-based nanoparticle technologies for liver targeting.Adv Drug Deliv Rev. 2020;154-155:79-101. doi: 10.1016/j.addr.2020.06.017. Epub 2020 Jun 20. Adv Drug Deliv Rev. 2020. PMID: 32574575 Review.
-
Alcohol use disorder and the liver.Addiction. 2021 May;116(5):1270-1278. doi: 10.1111/add.15204. Epub 2020 Aug 21. Addiction. 2021. PMID: 32710592
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
Cited by
-
Portal Fibrosis and the Ductular Reaction: Pathophysiological Role in the Progression of Liver Disease and Translational Opportunities.Gastroenterology. 2025 Apr;168(4):675-690. doi: 10.1053/j.gastro.2024.07.044. Epub 2024 Sep 7. Gastroenterology. 2025. PMID: 39251168 Review.
-
Plant extracts with antioxidant and hepatoprotective benefits for liver health: A bibliometric analysis of drug delivery systems.World J Gastroenterol. 2025 May 14;31(18):105836. doi: 10.3748/wjg.v31.i18.105836. World J Gastroenterol. 2025. PMID: 40496363 Free PMC article.
-
Chitosan-Based Nanoparticles Targeted Delivery System: In Treatment Approach for Dyslipidemia.Int J Nanomedicine. 2025 May 24;20:6611-6636. doi: 10.2147/IJN.S517492. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40438188 Free PMC article. Review.
-
Targeting Lipophagy in Liver Diseases: Impact on Oxidative Stress and Steatohepatitis.Antioxidants (Basel). 2025 Jul 24;14(8):908. doi: 10.3390/antiox14080908. Antioxidants (Basel). 2025. PMID: 40867807 Free PMC article. Review.
-
Navigating liver cancer: Precision targeting for enhanced treatment outcomes.Drug Deliv Transl Res. 2025 Jun;15(6):1935-1961. doi: 10.1007/s13346-024-01780-x. Epub 2025 Jan 23. Drug Deliv Transl Res. 2025. PMID: 39847205 Review.
References
-
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–168. - PubMed
-
- Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. - PubMed
-
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. - PubMed
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018;69:154–181. - PubMed
-
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

