A novel approach for the prevention of ionizing radiation-induced bone loss using a designer multifunctional cerium oxide nanozyme
- PMID: 36185749
- PMCID: PMC9507991
- DOI: 10.1016/j.bioactmat.2022.09.011
A novel approach for the prevention of ionizing radiation-induced bone loss using a designer multifunctional cerium oxide nanozyme
Abstract
The disability, mortality and costs due to ionizing radiation (IR)-induced osteoporotic bone fractures are substantial and no effective therapy exists. Ionizing radiation increases cellular oxidative damage, causing an imbalance in bone turnover that is primarily driven via heightened activity of the bone-resorbing osteoclast. We demonstrate that rats exposed to sublethal levels of IR develop fragile, osteoporotic bone. At reactive surface sites, cerium ions have the ability to easily undergo redox cycling: drastically adjusting their electronic configurations and versatile catalytic activities. These properties make cerium oxide nanomaterials fascinating. We show that an engineered artificial nanozyme composed of cerium oxide, and designed to possess a higher fraction of trivalent (Ce3+) surface sites, mitigates the IR-induced loss in bone area, bone architecture, and strength. These investigations also demonstrate that our nanozyme furnishes several mechanistic avenues of protection and selectively targets highly damaging reactive oxygen species, protecting the rats against IR-induced DNA damage, cellular senescence, and elevated osteoclastic activity in vitro and in vivo. Further, we reveal that our nanozyme is a previously unreported key regulator of osteoclast formation derived from macrophages while also directly targeting bone progenitor cells, favoring new bone formation despite its exposure to harmful levels of IR in vitro. These findings open a new approach for the specific prevention of IR-induced bone loss using synthesis-mediated designer multifunctional nanomaterials.
Keywords: ALP, Alkaline phosphatase; BMSC, Bone marrow derived mesenchymal stem cells; Bone resorption; Bone strength; CAT, Catalase; COLI, Collagen type I; CTSK, Cathepsin K; CTX-1, Cross-linked C-telopeptide of type I collagen; CeONPs, Cerium oxide nanoparticles; Cerium oxide; DFT, Density functional theory; DNA, Deoxyribonucleic acid; EPR, Electron paramagnetic resonance; FDA, Food and Drug Administration; GPX, Glutathione peroxidase; Gy, Gray; HIF1α, Hypoxia-inducible factor 1 alpha; IL-1β, Interleukin 1 beta; IL-6, Interleukin 6; IR, Ionizing radiation; Ionizing radiation; MNGC, Multinucleated giant cell; Nanozyme; OCN, Osteocalcin; Osteoporosis; RANKL, Receptor activator of nuclear factor kappa-Β ligand; ROS, Reactive oxygen species; SAED, Selected area electron diffraction; SOD, Superoxide dismutase; TRAP, Tartrate-resistant acid phosphatase; XPS, X-ray photoelectron spectroscopy.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
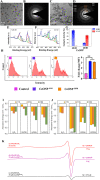
Figures






References
-
- Kim H.J., Boland P.J., Meredith D.S., Lis E., Zhang Z., Shi W., Yamada Y.J., Goodman K.A. Fractures of the sacrum after chemoradiation for rectal carcinoma: incidence, risk factors, and radiographic evaluation. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(No. 3) doi: 10.1016/j.ijrobp.2012.01.021. - DOI - PubMed
-
- Aoki M., Sato M., Hirose K., Akimoto H., Kawaguchi H., Hatayama Y., Ono S., Takai Y. Radiation-induced rib fracture after stereotactic body radiotherapy with a total dose of 54-56 Gy given in 9-7 fractions for patients with peripheral lung tumor: impact of maximum dose and fraction size. Radiat. Oncol. 2015;10 doi: 10.1186/s13014-015-0406-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

