Immunotherapy approaches for the treatment of diffuse midline gliomas
- PMID: 36185807
- PMCID: PMC9519005
- DOI: 10.1080/2162402X.2022.2124058
Immunotherapy approaches for the treatment of diffuse midline gliomas
Abstract
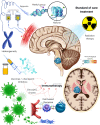
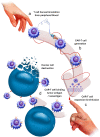
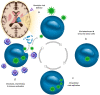
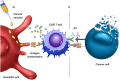
Diffuse midline gliomas (DMG) are a highly aggressive and universally fatal subgroup of pediatric tumors responsible for the majority of childhood brain tumor deaths. Median overall survival is less than 12 months with a 90% mortality rate at 2 years from diagnosis. Research into the underlying tumor biology and numerous clinical trials have done little to change the invariably poor prognosis. Continued development of novel, efficacious therapeutic options for DMGs remains a critically important area of active investigation. Given that DMGs are not amenable to surgical resection, have only limited response to radiation, and are refractory to traditional chemotherapy, immunotherapy has emerged as a promising alternative treatment modality. This review summarizes the various immunotherapy-based treatments for DMG as well as their specific limitations. We explore the use of cell-based therapies, oncolytic virotherapy or immunovirotherapy, immune checkpoint inhibition, and immunomodulatory vaccination strategies, and highlight the recent clinical success of anti-GD2 CAR-T therapy in diffuse intrinsic pontine glioma (DIPG) patients. Finally, we address the challenges faced in translating preclinical and early phase clinical trial data into effective standardized treatment for DMG patients.
Keywords: Virotherapy; cell-based therapy; diffuse intrinsic pontine glioma (DIPG); diffuse midline gliomas (DMG); glioma; immune checkpoint inhibition (ICI); immunotherapy; pediatric neuro-oncology; pediatric neurosurgery; vaccination.
© 2022 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
J.D.B. has an equity position in Treovir LLC, an oHSV clinical stage company and is a member of the POCKiT Diagnostics Board of Scientific Advisors. M.G.F. is a consultant for Twentyeight-Seven, Inc., and Blueprint Medicines Corporation. The remaining authors declared that no conflict of interest exists.
Figures
References
-
- Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, Stearns DS, Wolff JE, Wolinsky Y, Letterio JJ.. Alex’s lemonade stand foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2015;16(Suppl 10):x1–17. doi:10.1093/neuonc/nou327. - DOI - PMC - PubMed
-
- Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21(Supplement_5):v1–v100. doi:10.1093/neuonc/noz150. - DOI - PMC - PubMed
-
- Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, Baugh J, Chaney B, Hoffmann M, Lane A, Fuller C, Miles L, Hawkins C. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse Intrinsic pontine glioma (DIPG): a collaborative report from the international and european society for pediatric oncology DIPG registries. J Clin Oncol. 2018;36(19):1963–1972. doi:10.1200/JCO.2017.75.9308. - DOI - PMC - PubMed
-
- Gururangan S, McLaughlin CA, Brashears J, Watral MA, Provenzale J, Coleman RE, Halperin EC, Quinn J, Reardon D, Vredenburgh J, et al. Incidence and patterns of neuraxis metastases in children with diffuse pontine glioma. J Neurooncol. 2006;77(2):207–212. doi:10.1007/s11060-005-9029-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
