Wearable Bioelectronics for Chronic Wound Management
- PMID: 36186921
- PMCID: PMC9518812
- DOI: 10.1002/adfm.202111022
Wearable Bioelectronics for Chronic Wound Management
Abstract
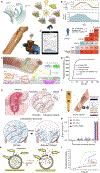
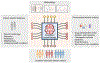
Chronic wounds are a major healthcare issue and can adversely affect the lives of millions of patients around the world. The current wound management strategies have limited clinical efficacy due to labor-intensive lab analysis requirements, need for clinicians' experiences, long-term and frequent interventions, limiting therapeutic efficiency and applicability. The growing field of flexible bioelectronics enables a great potential for personalized wound care owing to its advantages such as wearability, low-cost, and rapid and simple application. Herein, recent advances in the development of wearable bioelectronics for monitoring and management of chronic wounds are comprehensively reviewed. First, the design principles and the key features of bioelectronics that can adapt to the unique wound milieu features are introduced. Next, the current state of wound biosensors and on-demand therapeutic systems are summarized and highlighted. Furthermore, we discuss the design criteria of the integrated closed loop devices. Finally, the future perspectives and challenges in wearable bioelectronics for wound care are discussed.
Keywords: bioelectronics; biosensors; drug delivery; wearable devices; wound healing.
Figures









References
-
- Li D, Kular L, Vij M, Herter EK, Li X, Wang A, Chu T, Toma MA, Zhang L, Liapi E, Mota A, Blomqvist L, Gallais Sérézal I, Rollman O, Wikstrom JD, Bienko M, Berglund D, Ståhle M, Sommar P, Jagodic M, Landén NX, Proceedings of the National Academy of Sciences of the United States of America 2019, 116, 9443. - PMC - PubMed
-
- McLister A, McHugh J, Cundell J, Davis J, Advanced Materials 2016, 28, 5732. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
