Systemic gene therapy for methylmalonic acidemia using the novel adeno-associated viral vector 44.9
- PMID: 36186952
- PMCID: PMC9490190
- DOI: 10.1016/j.omtm.2022.09.001
Systemic gene therapy for methylmalonic acidemia using the novel adeno-associated viral vector 44.9
Abstract
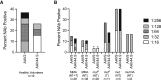
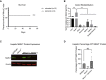
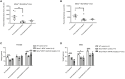
Methylmalonic acidemia (MMA) is a severe and potentially lethal autosomal recessive inborn error of metabolism most frequently caused by mutations in the methylmalonyl-CoA mutase (MMUT) gene. Proof-of-concept adeno-associated virus (AAV) gene therapy studies using mouse models of MMA have demonstrated promise for this therapeutic approach but translation to the clinic could be limited by preexisting capsid immunity and vector potency. Here we explore the efficacy of a novel clade E capsid, 44.9, as a serotype for systemic AAV gene therapy for MMA. An anti-AAV44.9 neutralizing antibody (NAb) survey in adult volunteers (n = 19) and a large cohort of MMA patients (n = 48) revealed a seroprevalence rate of ∼26% and 13%, respectively. The efficacy of AAV44.9 gene delivery was examined in two murine models of MMA, representing neonatal lethal and juvenile phenotypes of MMA. Systemic delivery of the AAV44.9-Mmut vector prevented lethality and lowered disease-related metabolites in MMA mice. Tissue biodistribution and transgene expression studies in treated MMA mice showed that AAV44.9 was efficient at transducing the liver and heart. In summary, we establish that AAV44.9 exhibits a low prevalence of preexisting NAb in humans, is highly efficacious in the treatment of clinically severe MMA mouse models and is therefore a promising vector for clinical translation.
Keywords: AAV 44.9; MMA; liver directed gene therapy; methylmalonic acidemia; neutralizing antibodies; systemic gene therapy.
© 2022.
Conflict of interest statement
R.J.C., G.D., J.A.C., and C.P.V. are inventors on a patent application filed by NIH on their behalf on the use of AAV44.9 as a gene therapy vector to treat MMA.
Figures


References
-
- Manoli I., Sloan J.L., Venditti C.P. In: Gene Reviews((R)) Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Mirzaa G.M., Amemiya A., editors. 1993. Isolated methylmalonic acidemia.
-
- Hörster F., Baumgartner M.R., Viardot C., Suormala T., Burgard P., Fowler B., Hoffmann G.F., Garbade S.F., Kölker S., Baumgartner E.R. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-cblA, cblB) Pediatr. Res. 2007;62:225–230. doi: 10.1203/PDR.0b013e3180a0325f. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

