Temperature analysis of 3D-printed biomaterials during unipolar and bipolar radiofrequency ablation procedure
- PMID: 36186978
- PMCID: PMC9515363
- DOI: 10.3389/fcvm.2022.978333
Temperature analysis of 3D-printed biomaterials during unipolar and bipolar radiofrequency ablation procedure
Abstract
Background: Due to their mechanical properties, the MED625FLX and TPU95A could be appropriate candidates for cardiac 3D surgical guide use during radiofrequency ablation (RFA) treatment.
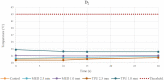
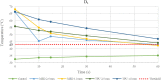
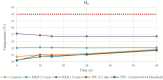
Methods: RFA aims to destroy the heart tissue, which cause arrhythmias, by applying a radiofrequency (RF) energy at critical temperature above +50.0°C, where the thermal damage is considered not reversible. This study aims to analyze the biomaterials thermal properties with different thicknesses, by testing the response to bipolar and unipolar RFA on porcine muscle samples (PMS), expressed in temperature. For the materials evaluation, the tissue temperature during RFA applications was recorded, firstly without (control) and after with the biomaterials in position. The biomaterials were considered suitable for the RFA treatment if: (1) the PMS temperatures with the samples were not statistically different compared with the control; (2) the temperatures never reached the threshold; (3) no geometrical changes after RFA were observed.
Results: Based on these criteria, none of the tested biomaterials resulted appropriate for unipolar RFA and the TPU95A failed almost all thermal tests also with the bipolar RFA. The 1.0 mm MED625FLX was modified by bipolar RFA in shape, losing its function. Instead, the 2.5 mm MED625FLX was considered suitable for bipolar RFA catheter use only.
Conclusions: The 2.5 mm MED625FLX could be used, in the design of surgical guides for RFA bipolar catheter only, because of mechanical, geometrical, and thermal properties. None of biomaterials tested are appropriate for unipolar ablation catheter because of temperature concerns. Further investigations for clinical use are eagerly awaited.
Keywords: 3D surgical guide; arrhythmias treatment; biomaterial tests; radiofrequency ablation; thermal test.
Copyright © 2022 Cappello, Candelari, Pannone, Monaco, Bori, Talevi, Ramak, La Meir, Gharaviri, Chierchia, Innocenti and de Asmundis.
Conflict of interest statement
Author ML is consultant for Atricure. GC received compensation for teaching purposes and proctoring from Medtronic, Abbott, Biotronik, Boston Scientific, Acutus Medical. CA receives research grants on behalf of the center from Biotronik, Medtronic, Abbott, LivaNova, Boston Scientific, AtriCure, Philips, and Acutus; received compensation for teaching purposes and proctoring from Medtronic, Abbott, Biotronik, Livanova, Boston Scientific, Atricure, Acutus Medical Daiichi Sankyo. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Stratasys. Available online at: www.stratasys.com (accessed May 24, 2022).
-
- Ultimaker. Available online at: www.ultimaker.com (accessed May 24, 2022).
LinkOut - more resources
Full Text Sources

