Determinants of the time-to-peak left ventricular dP/dt (Td) and QRS duration with different fusion strategies in cardiac resynchronization therapy
- PMID: 36186985
- PMCID: PMC9520326
- DOI: 10.3389/fcvm.2022.979581
Determinants of the time-to-peak left ventricular dP/dt (Td) and QRS duration with different fusion strategies in cardiac resynchronization therapy
Abstract
Background: Cardiac resynchronization therapy (CRT) is helpful in selected patients; however, responder rates rarely exceed 70%. Optimization of CRT may therefore benefit a large number of patients. Time-to-peak dP/dt (Td) is a novel marker of myocardial synergy that reflects the degree of myocardial dyssynchrony with the potential to guide and optimize treatment with CRT. Optimal electrical activation is a prerequisite for CRT to be effective. Electrical activation can be altered by changing the electrical wave-front fusion resulting from pacing to optimize resynchronization. We designed this study to understand the acute effects of different electrical wave-front fusion strategies and LV pre-/postexcitation on Td and QRS duration (QRSd). A better understanding of measuring and optimizing resynchronization can help improve the benefits of CRT.
Methods: Td and QRSd were measured in 19 patients undergoing a CRT implantation. Two biventricular pacing groups were compared: pacing the left ventricle (LV) with fusion with intrinsic right ventricular activation (FUSION group) and pacing the LV and right ventricle (RV) at short atrioventricular delay (STANDARD group) to avoid fusion with intrinsic RV activation. A quadripolar LV lead enabled pacing from widely separated electrodes; distal (DIST), proximal (PROX) and both electrodes combined (multipoint pacing, MPP). The LV was stimulated relative in time to RV activation (either RV pace-onset or QRS-onset), with the LV stimulated prior to (PRE), simultaneous with (SIM) or after (POST) RV activation. In addition, we analyzed the interactions of the two groups (FUSION/STANDARD) with three different electrode configurations (DIST, PROX, MPP), each paced with three different degrees of LV pre-/postexcitation (PRE, SIM, POST) in a statistical model.
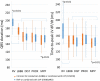
Results: We found that FUSION provided shorter Td and QRSd than STANDARD, MPP provided shorter Td and QRSd than DIST and PROX, and SIM provided both the shortest QRSd and Td compared to PRE and POST. The interaction analysis revealed that pacing MPP with fusion with intrinsic RV activation simultaneous with the onset of the QRS complex (MPP*FUSION*SIM) shortened QRSd and Td the most compared to all other modes and configurations. The difference in QRSd and Td from their respective references were significantly correlated (β = 1, R = 0.9, p < 0.01).
Conclusion: Pacing modes and electrode configurations designed to optimize electrical wave-front fusion (intrinsic RV activation, LV multipoint pacing and simultaneous RV and LV activation) shorten QRSd and Td the most. As demonstrated in this study, electrical and mechanical measures of resynchronization are highly correlated. Therefore, Td can potentially serve as a marker for CRT optimization.
Keywords: LV dP/dtmax; QRS duration; acute hemodynamic response; cardiac resynchronization therapy; fusion with native conduction; heart failure.
Copyright © 2022 Odland, Holm, Cornelussen and Kongsgård.
Conflict of interest statement
Author RC was employed by Medtronic. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Okafor O, Zegard A, van Dam P, Stegemann B, Qiu T, Marshall H, et al. Changes in QRS area and QRS duration after cardiac resynchronization therapy predict cardiac mortality, heart failure hospitalizations, and ventricular arrhythmias. J Am Heart Assoc. (2019) 8:e013539. 10.1161/jaha.119.013539 - DOI - PMC - PubMed
-
- Stankovic I, Stefanovic M, Prinz C, Ciarka A, Daraban AM, Kotrc M, et al. The association of mechanical dyssynchrony and resynchronization therapy with survival in heart failure with a wide QRS complex: a two-world study. Int J Cardiovasc Imaging. (2020) 36:1507–14. 10.1007/s10554-020-01865-x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials

