IL-12p40 deletion aggravates lipopolysaccharide-induced cardiac dysfunction in mice
- PMID: 36186987
- PMCID: PMC9523082
- DOI: 10.3389/fcvm.2022.950029
IL-12p40 deletion aggravates lipopolysaccharide-induced cardiac dysfunction in mice
Abstract
Background: Cardiac dysfunction is one of the most common complications of sepsis and is associated with the adverse outcomes and high mortality of sepsis patients. IL-12p40, the common subunit of IL-12 and IL-23, has been shown to be involved in a variety of inflammation-related diseases, such as psoriasis and inflammatory bowel disease. However, the role of IL-12p40 in lipopolysaccharide (LPS)-induced cardiac dysfunction remains obscure. This study aimed to explore the role of IL-12p40 in LPS-induced cardiac dysfunction and its potential mechanisms.
Methods: In this study, mice were treated with LPS and the cardiac expression of IL-12p40 was determined. Then, IL-12p40-/- mice were used to detect the role and mechanisms of IL-12p40 in LPS-induced cardiac injury. In addition, monocytes were adoptively transferred to IL-12p40-/- mice to explore their effects on LPS-induced cardiac dysfunction.
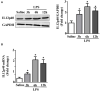
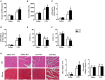
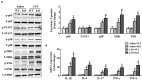
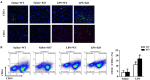
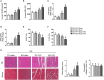
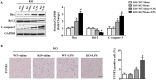
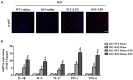
Results: The results showed that cardiac IL-12p40 expression was significantly increased after treated with LPS. In addition, IL-12p40 deletion significantly aggravated LPS-induced cardiac dysfunction, evidenced by the increased serum levels of cardiomyocyte injury markers and heart injury scores, as well as by the deteriorated cardiac function. Moreover, IL-12p40 deletion increased LPS-induced monocyte accumulation and cardiac expression of inflammatory cytokines, as well as enhanced the activation of the NF-κB and MAPK pathways. Furthermore, adoptive transfer WT mouse monocytes to IL-12p40-/- mice alleviated LPS-induced cardiac dysfunction and decreased the phosphorylation of p65.
Conclusion: IL-12p40 deletion significantly aggravated LPS-induced cardiac injury and cardiac dysfunction in mice by regulating the NF-κB and MAPK signaling pathways, and this process was related to monocytes. Therefore, IL-12p40 show a protective role in SIC, and IL-12p40 deficiency or anti-IL-12p40 monoclonal antibodies may be detrimental to patients with SIC.
Keywords: IL-12p40 deletion; LPS; cardiac dysfunction; monocytes; sepsis.
Copyright © 2022 Liu, Wang, Zhang, Ye, Wang, Xu, Zhao, Feng, Lu, Pan, Pan, Wei, Tian, Li, Lyu, Ye and Wan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Il12a Deletion Aggravates Sepsis-Induced Cardiac Dysfunction by Regulating Macrophage Polarization.Front Pharmacol. 2021 Jul 2;12:632912. doi: 10.3389/fphar.2021.632912. eCollection 2021. Front Pharmacol. 2021. PMID: 34276358 Free PMC article.
-
Interleukin-5 deletion promotes sepsis-induced M1 macrophage differentiation, deteriorates cardiac dysfunction, and exacerbates cardiac injury via the NF-κB p65 pathway in mice.Biofactors. 2020 Nov;46(6):1006-1017. doi: 10.1002/biof.1681. Epub 2020 Oct 11. Biofactors. 2020. PMID: 33043521
-
STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells.Blood. 2005 Jan 15;105(2):689-96. doi: 10.1182/blood-2004-04-1309. Epub 2004 Jul 13. Blood. 2005. PMID: 15251981
-
Dexamethasone inhibits IL-12p40 production in lipopolysaccharide-stimulated human monocytic cells by down-regulating the activity of c-Jun N-terminal kinase, the activation protein-1, and NF-kappa B transcription factors.J Immunol. 2004 Jan 1;172(1):318-30. doi: 10.4049/jimmunol.172.1.318. J Immunol. 2004. PMID: 14688340
-
[Protective effect of TAK242 blocking Toll-like receptor 4 pathway on septic myocardial injury and cardiac dysfunction].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021 Oct;33(10):1226-1231. doi: 10.3760/cma.j.cn121430-20210620-00915. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021. PMID: 34955133 Chinese.
Cited by
-
Pathogenesis and treatment strategies of sepsis-induced myocardial injury: modern and traditional medical perspectives.Int J Biol Sci. 2025 May 15;21(8):3478-3504. doi: 10.7150/ijbs.111288. eCollection 2025. Int J Biol Sci. 2025. PMID: 40520010 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

