Automated assessment of breast margins in deep ultraviolet fluorescence images using texture analysis
- PMID: 36187258
- PMCID: PMC9484420
- DOI: 10.1364/BOE.464547
Automated assessment of breast margins in deep ultraviolet fluorescence images using texture analysis
Abstract
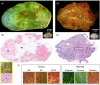
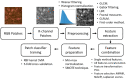
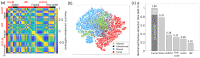
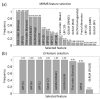
Microscopy with ultraviolet surface excitation (MUSE) is increasingly studied for intraoperative assessment of tumor margins during breast-conserving surgery to reduce the re-excision rate. Here we report a two-step classification approach using texture analysis of MUSE images to automate the margin detection. A study dataset consisting of MUSE images from 66 human breast tissues was constructed for model training and validation. Features extracted using six texture analysis methods were investigated for tissue characterization, and a support vector machine was trained for binary classification of image patches within a full image based on selected feature subsets. A weighted majority voting strategy classified a sample as tumor or normal. Using the eight most predictive features ranked by the maximum relevance minimum redundancy and Laplacian scores methods has achieved a sample classification accuracy of 92.4% and 93.0%, respectively. Local binary pattern alone has achieved an accuracy of 90.3%.
© 2022 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
The authors have no conflicts of interest to claim.
Figures





References
-
- Nash R., Goodman M., Lin C. C., Freedman R. A., Dominici L. S., Ward K., Jemal A., “State variation in the receipt of a contralateral prophylactic mastectomy among women who received a diagnosis of invasive unilateral early-stage breast cancer in the United States, 2004-2012,” JAMA Surg. 152(7), 648–657 (2017).10.1001/jamasurg.2017.0115 - DOI - PMC - PubMed
-
- Kantor O., Pesce C., Kopkash K., Barrera E., Winchester D. J., Kuchta K., Yao K., “Impact of the Society of Surgical Oncology-American Society for Radiation Oncology margin guidelines on breast-conserving surgery and mastectomy trends,” J Am. Coll. Surg. 229(1), 104–114 (2019).10.1016/j.jamcollsurg.2019.02.051 - DOI - PubMed
LinkOut - more resources
Full Text Sources
