Role of the renin-angiotensin system in the development of COVID-19-associated neurological manifestations
- PMID: 36187294
- PMCID: PMC9523599
- DOI: 10.3389/fncel.2022.977039
Role of the renin-angiotensin system in the development of COVID-19-associated neurological manifestations
Abstract
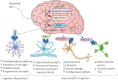
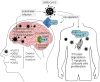
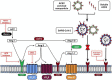
SARS-CoV-2 causes COVID-19, which has claimed millions of lives. This virus can infect various cells and tissues, including the brain, for which numerous neurological symptoms have been reported, ranging from mild and non-life-threatening (e.g., headaches, anosmia, dysgeusia, and disorientation) to severe and life-threatening symptoms (e.g., meningitis, ischemic stroke, and cerebral thrombosis). The cellular receptor for SARS-CoV-2 is angiotensin-converting enzyme 2 (ACE2), an enzyme that belongs to the renin-angiotensin system (RAS). RAS is an endocrine system that has been classically associated with regulating blood pressure and fluid and electrolyte balance; however, it is also involved in promoting inflammation, proliferation, fibrogenesis, and lipogenesis. Two pathways constitute the RAS with counter-balancing effects, which is the key to its regulation. The first axis (classical) is composed of angiotensin-converting enzyme (ACE), angiotensin (Ang) II, and angiotensin type 1 receptor (AT1R) as the main effector, which -when activated- increases the production of aldosterone and antidiuretic hormone, sympathetic nervous system tone, blood pressure, vasoconstriction, fibrosis, inflammation, and reactive oxygen species (ROS) production. Both systemic and local classical RAS' within the brain are associated with cognitive impairment, cell death, and inflammation. The second axis (non-classical or alternative) includes ACE2, which converts Ang II to Ang-(1-7), a peptide molecule that activates Mas receptor (MasR) in charge of opposing Ang II/AT1R actions. Thus, the alternative RAS axis enhances cognition, synaptic remodeling, cell survival, cell signal transmission, and antioxidant/anti-inflammatory mechanisms in the brain. In a physiological state, both RAS axes remain balanced. However, some factors can dysregulate systemic and local RAS arms. The binding of SARS-CoV-2 to ACE2 causes the internalization and degradation of this enzyme, reducing its activity, and disrupting the balance of systemic and local RAS, which partially explain the appearance of some of the neurological symptoms associated with COVID-19. Therefore, this review aims to analyze the role of RAS in the development of the neurological effects due to SARS-CoV-2 infection. Moreover, we will discuss the RAS-molecular targets that could be used for therapeutic purposes to treat the short and long-term neurological COVID-19-related sequelae.
Keywords: ACE2; COVID-19; SARS-CoV-2; long COVID; neuroinflammation; neurological manifestations; renin-angiotensin system.
Copyright © 2022 Méndez-García, Escobedo, Minguer-Uribe, Viurcos-Sanabria, Aguayo-Guerrero, Carrillo-Ruiz and Solleiro-Villavicencio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Disequilibrium between the classic renin-angiotensin system and its opposing arm in SARS-CoV-2-related lung injury.Am J Physiol Lung Cell Mol Physiol. 2020 Aug 1;319(2):L325-L336. doi: 10.1152/ajplung.00189.2020. Epub 2020 Jul 8. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32639866 Free PMC article. Review.
-
Brain Renin-Angiotensin System: From Physiology to Pathology in Neuronal Complications Induced by SARS-CoV-2.Anal Cell Pathol (Amst). 2023 Aug 4;2023:8883492. doi: 10.1155/2023/8883492. eCollection 2023. Anal Cell Pathol (Amst). 2023. PMID: 37575318 Free PMC article. Review.
-
Insights on SARS-CoV-2 Molecular Interactions With the Renin-Angiotensin System.Front Cell Dev Biol. 2020 Sep 16;8:559841. doi: 10.3389/fcell.2020.559841. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33042994 Free PMC article. Review.
-
Repressed Ang 1-7 in COVID-19 Is Inversely Associated with Inflammation and Coagulation.mSphere. 2022 Aug 31;7(4):e0022022. doi: 10.1128/msphere.00220-22. Epub 2022 Aug 1. mSphere. 2022. PMID: 35913134 Free PMC article.
-
Angiotensin II Type I Receptor (AT1R): The Gate towards COVID-19-Associated Diseases.Molecules. 2022 Mar 22;27(7):2048. doi: 10.3390/molecules27072048. Molecules. 2022. PMID: 35408447 Free PMC article. Review.
Cited by
-
Relationships between fatigue severity scale (FSS)/ scale for mood assessment (EVEA) and clinical manifestations in spanish long-COVID patients.PLoS One. 2025 Jul 7;20(7):e0324075. doi: 10.1371/journal.pone.0324075. eCollection 2025. PLoS One. 2025. PMID: 40622937 Free PMC article.
-
The contribution of gut-brain axis to development of neurological symptoms in COVID-19 recovered patients: A hypothesis and review of literature.Front Cell Infect Microbiol. 2022 Dec 22;12:983089. doi: 10.3389/fcimb.2022.983089. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36619768 Free PMC article. Review.
-
Role of the Renin-Angiotensin System in Long COVID's Cardiovascular Injuries.Biomedicines. 2023 Jul 15;11(7):2004. doi: 10.3390/biomedicines11072004. Biomedicines. 2023. PMID: 37509643 Free PMC article. Review.
-
Ketogenic Diet and Ketone Bodies as Clinical Support for the Treatment of SARS-CoV-2-Review of the Evidence.Viruses. 2023 May 27;15(6):1262. doi: 10.3390/v15061262. Viruses. 2023. PMID: 37376562 Free PMC article. Review.
-
Cerebral small vessel disease pathology in COVID-19 patients: A systematic review.Ageing Res Rev. 2023 Jul;88:101962. doi: 10.1016/j.arr.2023.101962. Epub 2023 May 22. Ageing Res Rev. 2023. PMID: 37224885 Free PMC article.
References
-
- Abrahão M. V., Dos Santos N. F. T., Kuwabara W. M. T., do Amaral F. G., do Carmo, Buonfiglio D., et al. (2019). Identification of insulin-regulated aminopeptidase (IRAP) in the rat pineal gland and the modulation of melatonin synthesis by angiotensin IV. Brain Res. 1704 40–46. 10.1016/j.brainres.2018.09.015 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

