The Mitochondrial Unfolded Protein Response: A Novel Protective Pathway Targeting Cardiomyocytes
- PMID: 36187338
- PMCID: PMC9519344
- DOI: 10.1155/2022/6430342
The Mitochondrial Unfolded Protein Response: A Novel Protective Pathway Targeting Cardiomyocytes
Abstract
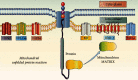
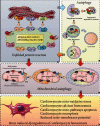
Mitochondrial protein homeostasis in cardiomyocyte injury determines not only the normal operation of mitochondrial function but also the fate of mitochondria in cardiomyocytes. Studies of mitochondrial protein homeostasis have become an integral part of cardiovascular disease research. Modulation of the mitochondrial unfolded protein response (UPRmt), a protective factor for cardiomyocyte mitochondria, may in the future become an important treatment strategy for myocardial protection in cardiovascular disease. However, because of insufficient understanding of the UPRmt and inadequate elucidation of relevant mechanisms, few therapeutic drugs targeting the UPRmt have been developed. The UPRmt maintains a series of chaperone proteins and proteases and is activated when misfolded proteins accumulate in the mitochondria. Mitochondrial injury leads to metabolic dysfunction in cardiomyocytes. This paper reviews the relationship of the UPRmt and mitochondrial quality monitoring with cardiomyocyte protection. This review mainly introduces the regulatory mechanisms of the UPRmt elucidated in recent years and the relationship between the UPRmt and mitophagy, mitochondrial fusion/fission, mitochondrial biosynthesis, and mitochondrial energy metabolism homeostasis in order to generate new ideas for the study of the mitochondrial protein homeostasis mechanisms as well as to provide a reference for the targeted drug treatment of imbalances in mitochondrial protein homeostasis following cardiomyocyte injury.
Copyright © 2022 Jinfeng Liu et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

