Live imaging of echinoderm embryos to illuminate evo-devo
- PMID: 36187474
- PMCID: PMC9521734
- DOI: 10.3389/fcell.2022.1007775
Live imaging of echinoderm embryos to illuminate evo-devo
Abstract
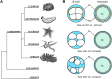
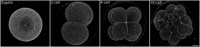
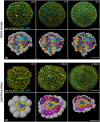
Echinoderm embryos have been model systems for cell and developmental biology for over 150 years, in good part because of their optical clarity. Discoveries that shaped our understanding of fertilization, cell division and cell differentiation were only possible because of the transparency of sea urchin eggs and embryos, which allowed direct observations of intracellular structures. More recently, live imaging of sea urchin embryos, coupled with fluorescence microscopy, has proven pivotal to uncovering mechanisms of epithelial to mesenchymal transition, cell migration and gastrulation. However, live imaging has mainly been performed on sea urchin embryos, while echinoderms include numerous experimentally tractable species that present interesting variation in key aspects of morphogenesis, including differences in embryo compaction and mechanisms of blastula formation. The study of such variation would allow us not only to understand how tissues are formed in echinoderms, but also to identify which changes in cell shape, cell-matrix and cell-cell contact formation are more likely to result in evolution of new embryonic shapes. Here we argue that adapting live imaging techniques to more echinoderm species will be fundamental to exploit such an evolutionary approach to the study of morphogenesis, as it will allow measuring differences in dynamic cellular behaviors - such as changes in cell shape and cell adhesion - between species. We briefly review existing methods for live imaging of echinoderm embryos and describe in detail how we adapted those methods to allow long-term live imaging of several species, namely the sea urchin Lytechinus pictus and the sea stars Patiria miniata and Patiriella regularis. We outline procedures to successfully label, mount and image early embryos for 10-16 h, from cleavage stages to early blastula. We show that data obtained with these methods allows 3D segmentation and tracking of individual cells over time, the first step to analyze how cell shape and cell contact differ among species. The methods presented here can be easily adopted by most cell and developmental biology laboratories and adapted to successfully image early embryos of additional species, therefore broadening our understanding of the evolution of morphogenesis.
Keywords: echinoderm; live image; morphogenesis; sea star; sea urchin.
Copyright © 2022 Barone and Lyons.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Arnone M. I., Byrne M., Martinez P. (2015). Echinodermata. Evol. Dev. Biol. Invertebr. 6, 1–58. 10.1007/978-3-7091-1856-6_1 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

