Implications of High-Density Cholesterol Metabolism for Oocyte Biology and Female Fertility
- PMID: 36187480
- PMCID: PMC9518216
- DOI: 10.3389/fcell.2022.941539
Implications of High-Density Cholesterol Metabolism for Oocyte Biology and Female Fertility
Abstract
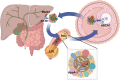
Cholesterol is an essential component of animal cells. Different regulatory mechanisms converge to maintain adequate levels of this lipid because both its deficiency and excess are unfavorable. Low cell cholesterol content promotes its synthesis and uptake from circulating lipoproteins. In contrast, its excess induces the efflux to high-density lipoproteins (HDL) and their transport to the liver for excretion, a process known as reverse cholesterol transport. Different studies suggest that an abnormal HDL metabolism hinders female fertility. HDL are the only lipoproteins detected in substantial amounts in follicular fluid (FF), and their size and composition correlate with embryo quality. Oocytes obtain cholesterol from cumulus cells via gap junctions because they cannot synthesize cholesterol de novo and lack HDL receptors. Recent evidence has supported the possibility that FF HDL play a major role in taking up excess unesterified cholesterol (UC) from the oocyte. Indeed, genetically modified mouse models with disruptions in reverse cholesterol transport, some of which show excessive circulating UC levels, exhibit female infertility. Cholesterol accumulation can affect the egg´s viability, as reported in other cell types, and activate the plasma membrane structure and activity of membrane proteins. Indeed, in mice deficient for the HDL receptor Scavenger Class B Type I (SR-B1), excess circulating HDL cholesterol and UC accumulation in oocytes impairs meiosis arrest and hinders the developmental capacity of the egg. In other cells, the addition of cholesterol activates calcium channels and dysregulates cell death/survival signaling pathways, suggesting that these mechanisms may link altered HDL cholesterol metabolism and infertility. Although cholesterol, and lipids in general, are usually not evaluated in infertile patients, one study reported high circulating UC levels in women showing longer time to pregnancy as an outcome of fertility. Based on the evidence described above, we propose the existence of a well-regulated and largely unexplored system of cholesterol homeostasis controlling traffic between FF HDL and oocytes, with significant implications for female fertility.
Keywords: cholesterol; female fertility; high-density lipoprotein metabolism; oocyte; unesterified cholesterol.
Copyright © 2022 Arias, Quiroz, Santander, Morselli and Busso.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

