Spectrum of Movement Disorders in Niemann-Pick Disease Type C
- PMID: 36187872
- PMCID: PMC9479749
- DOI: 10.5334/tohm.701
Spectrum of Movement Disorders in Niemann-Pick Disease Type C
Abstract
Introduction: Niemann-Pick disease type C (NPC) is an autosomal recessive neurovisceral lipid storage disorder caused by mutations in the NPC 1 or 2 genes. Movement disorders can occur as the first symptom and as predominant symptom mainly in juvenile-onset. The frequency and heterogeneity of movement disorders in NPC are not well described. We studied the frequency and spectrum of movement disorders in patients with NPC of different age of onset.
Methods: Retrospective chart review of patients with NPC diagnosed based on the Suspicion Index tool and demonstration of foamy macrophages/sea-blue histiocytes in bone marrow aspirate.
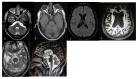
Results: We report 9 cases of NPC with 2 patients of late-infantile, 4 juvenile-onset and 3 of adult-onset. The mean age at onset of symptoms was 11.7 ± 10.4 (range 4-38 years) and the median duration of illness was 4 years. Vertical supranuclear gaze palsy (VSGP) was noted in 8 patients and VSGP with slowing of saccade in 1 patient. Splenomegaly was seen in 5 patients. Movement disorders as the first symptom occurred in 4 patients. Dystonia was the first symptom in 2 patients and cerebellar ataxia in 2 patients. Cerebellar ataxia occurred during the course of illness in 5 patients, dystonia in 6 patients. One patient with late-infantile NPC had stimulus-sensitive myoclonus.
Conclusion: Movement disorders are common in NPC and occur as a presenting symptom. Cerebellar ataxia and dystonia are the most common movement disorder in NPC. Vertical supranuclear gaze palsy along with the movement disorders should lead to clinical suspicion of NPC.
Keywords: Niemann-Pick Type C; cerebellar ataxia; dystonia; movement disorders; sphingolipidoses; supranuclear gaze palsy.
Copyright: © 2022 The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures


Similar articles
-
Spectrum of Movement Disorders of Late-Onset Niemann-Pick Disease Type C.Can J Neurol Sci. 2022 Nov;49(6):804-808. doi: 10.1017/cjn.2021.222. Epub 2021 Sep 16. Can J Neurol Sci. 2022. PMID: 34526163
-
Heterogeneity and frequency of movement disorders in juvenile and adult-onset Niemann-Pick C disease.J Neurol. 2014 Jan;261(1):174-9. doi: 10.1007/s00415-013-7159-9. Epub 2013 Nov 1. J Neurol. 2014. PMID: 24178705
-
[Adult onset Niemann-Pick type C disease and psychosis: literature review].Encephale. 2013 Oct;39(5):315-9. doi: 10.1016/j.encep.2013.04.013. Epub 2013 Aug 5. Encephale. 2013. PMID: 23928063 Review. French.
-
Clinical disease characteristics of patients with Niemann-Pick Disease Type C: findings from the International Niemann-Pick Disease Registry (INPDR).Orphanet J Rare Dis. 2022 Feb 14;17(1):51. doi: 10.1186/s13023-022-02200-4. Orphanet J Rare Dis. 2022. PMID: 35164809 Free PMC article.
-
The adult form of Niemann-Pick disease type C.Brain. 2007 Jan;130(Pt 1):120-33. doi: 10.1093/brain/awl260. Epub 2006 Sep 26. Brain. 2007. PMID: 17003072 Review.
Cited by
-
Global and Targeted Metabolomics for Revealing Metabolomic Alteration in Niemann-Pick Disease Type C Model Cells.Metabolites. 2024 Sep 24;14(10):515. doi: 10.3390/metabo14100515. Metabolites. 2024. PMID: 39452896 Free PMC article.
-
Role of Botulinum Toxin in Treatment of Secondary Dystonia: A Case Series and Overview of Literature.Toxins (Basel). 2024 Jun 24;16(7):286. doi: 10.3390/toxins16070286. Toxins (Basel). 2024. PMID: 39057926 Free PMC article. Review.
-
A Review of Ocular Movement Abnormalities in Hereditary Cerebellar Ataxias.Cerebellum. 2024 Apr;23(2):702-721. doi: 10.1007/s12311-023-01554-0. Epub 2023 Mar 31. Cerebellum. 2024. PMID: 37000369 Review.
-
Pediatric Genetic Dystonias: Current Diagnostic Approaches and Treatment Options.Life (Basel). 2025 Jun 20;15(7):992. doi: 10.3390/life15070992. Life (Basel). 2025. PMID: 40724495 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

