Bacterioplankton seasonality in deep high-mountain lakes
- PMID: 36187988
- PMCID: PMC9519062
- DOI: 10.3389/fmicb.2022.935378
Bacterioplankton seasonality in deep high-mountain lakes
Abstract
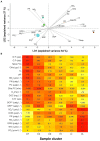
Due to global warming, shorter ice cover duration might drastically affect the ecology of lakes currently undergoing seasonal surface freezing. High-mountain lakes show snow-rich ice covers that determine contrasting conditions between ice-off and ice-on periods. We characterized the bacterioplankton seasonality in a deep high-mountain lake ice-covered for half a year. The lake shows a rich core bacterioplankton community consisting of three components: (i) an assemblage stable throughout the year, dominated by Actinobacteria, resistant to all environmental conditions; (ii) an ice-on-resilient assemblage dominating during the ice-covered period, which is more diverse than the other components and includes a high abundance of Verrucomicrobia; the deep hypolimnion constitutes a refuge for many of the typical under-ice taxa, many of which recover quickly during autumn mixing; and (iii) an ice-off-resilient assemblage, which members peak in summer in epilimnetic waters when the rest decline, characterized by a dominance of Flavobacterium, and Limnohabitans. The rich core community and low random elements compared to other relatively small cold lakes can be attributed to its simple hydrological network in a poorly-vegetated catchment, the long water-residence time (ca. 4 years), and the long ice-cover duration; features common to many headwater deep high-mountain lakes.
Keywords: Actinobacteria hgcl_clade; Flavobacterium; Limnohabitans; Verrucomicrobia; bacteria coexistence; core community; microbial ecology; under-ice ecology.
Copyright © 2022 Zufiaurre, Felip, Camarero, Sala-Faig, Juhanson, Bonilla-Rosso, Hallin and Catalan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Alonso-Sáez L., Gasol J. M., Lefort T., Hofer J., Sommaruga R. (2006). Effect of natural sunlight on bacterial activity and differential sensitivity of natural bacterioplankton groups in northwestern Mediterranean coastal waters. Appl. Environ. Microbiol. 72, 5806–5813. doi: 10.1128/AEM.00597-06, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

