Green Chromatographic Method for Determination of Active Pharmaceutical Ingredient, Preservative, and Antioxidant in an Injectable Formulation: Robustness by Design Expert
- PMID: 36188248
- PMCID: PMC9520538
- DOI: 10.1021/acsomega.2c03387
Green Chromatographic Method for Determination of Active Pharmaceutical Ingredient, Preservative, and Antioxidant in an Injectable Formulation: Robustness by Design Expert
Abstract
We report an efficient HPLC method for simultaneous qualitative and quantitative analysis of lincosamide antibiotic injectable formulations containing Clindamycin phosphate (CMN), benzyl alcohol (BA), and ethylenediaminetetraacetic acid (EDTA) as major ingredients. The three components were separated by Phenomenex prodigy C8 (250 mm × 4.6 mm, 5 μm) HPLC column, flow rate 1.1 mL/min, injection volume 30 μL, and column temperature 35 °C, using 0.05 M sodium acetate buffer (pH 4.5) with acetonitrile (ACN) in the ratio of 80:20 (v/v). The detection wavelength was set as 240 nm. The method was validated as per International Conference on Harmonization (ICH) guidelines and was confirmed to be specific, precise, accurate, and linear. Method robustness was executed by utilizing quality in the design of the experiment. Accuracy results were found to be 99.3-100.5% for CMN, 99.3-100.8% for BA, and 99.1-100.3% for EDTA. Precision results were obtained as % relative standard deviation (RSD): 0.6% for CMN, 0.4% for BA, and 0.4% for EDTA. Correlation coefficient (r 2) values were obtained as >0.999 for the three components. Analytical solutions are stable for 48 h at benchtop and refrigerator conditions. The greenness of the analytical method was evaluated by the Green Analytical Procedure Index (GAPI), National Environmental Method Index (NEMI), analytical eco-scale, and Analytical Greenness (AGREE) tools to confirm that the method is eco-friendly.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
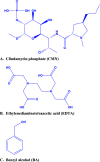
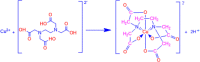
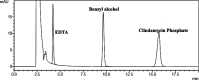

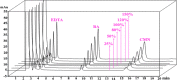


References
-
- The European agency for the evaluation of the medical products. https://www.ema.europa.eu/en/documents/scientific-guideline/note-guidanc.... (Accessed on October 7, 2021).
-
- The European agency for the evaluation of the medical products. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-ex.... (Accessed on October 7, 2021).
-
- USP 43 NF 38 General Chapters: ⟨341⟩ Antimicrobial agents-content; United States Pharmacopeial Convention, Rockville, Maryland, USA, 2020.
-
- ICH Harmonized Tripartite Guideline. Test procedures and acceptance criteria for new drug substances and new drug products: chemical substances Q6A, 1996.
-
- Khade V.; Mirgane S. High-performance liquid chromatography method for the analysis of Benzyl Alcohol. Int. J. Eng. Res. 2014, 5, 887–889.
LinkOut - more resources
Full Text Sources

