Visible Light-Promoted Green and Sustainable Approach for One-Pot Synthesis of 4,4'-(Arylmethylene)bis(1H-pyrazol-5-ols), In Vitro Anticancer Activity, and Molecular Docking with Covid-19 Mpro
- PMID: 36188265
- PMCID: PMC9520760
- DOI: 10.1021/acsomega.2c04506
Visible Light-Promoted Green and Sustainable Approach for One-Pot Synthesis of 4,4'-(Arylmethylene)bis(1H-pyrazol-5-ols), In Vitro Anticancer Activity, and Molecular Docking with Covid-19 Mpro
Abstract
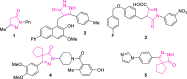
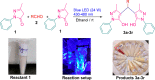
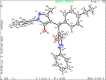
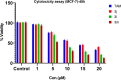
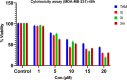
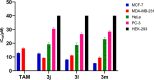
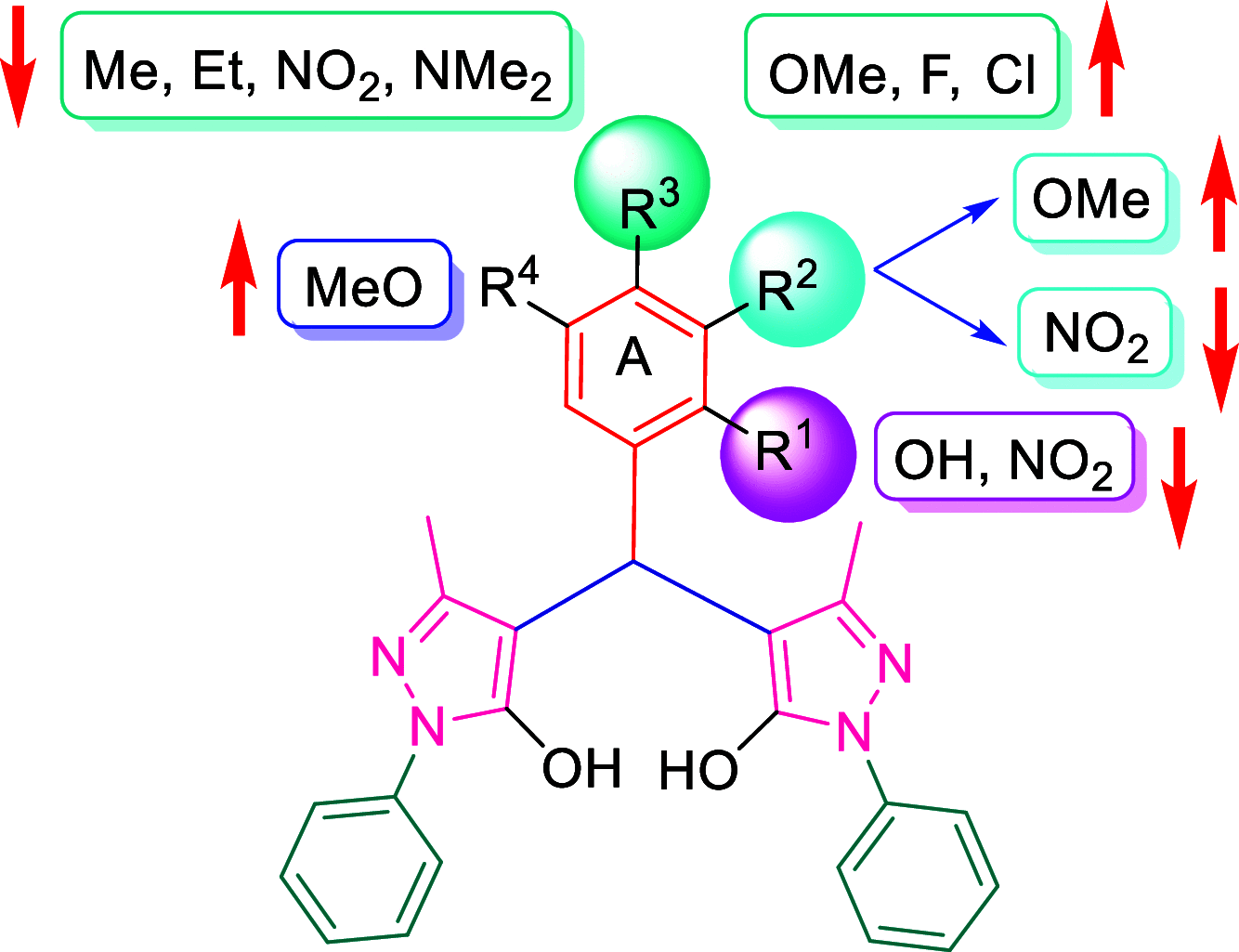
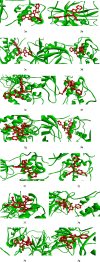
A visible light-promoted, efficient, green, and sustainable strategy has been adopted to unlatch a new pathway toward the synthesis of a library of medicinally important 4,4'-(arylmethylene)bis(1H-pyrazol-5-ols) moieties using substituted aromatic aldehydes and sterically hindered 3-methyl-1-phenyl-2-pyrazoline-5-one in excellent yield. This reaction shows high functional group tolerance and provides a cost-effective and catalyst-free protocol for the quick synthesis of biologically active compounds from readily available substrates. Synthesized compounds were characterized by spectroscopic techniques such as IR, 1HNMR, 13CNMR, and single-crystal XRD analysis. All the synthesized compounds were evaluated for their antiproliferative activities against a panel of five different human cancer cell lines and compared with Tamoxifen using MTT assay. Compound 3m exhibited maximum antiproliferative activity and was found to be more active as compared to Tamoxifen against both the MCF-7 and MDA-MB-231 cell lines with an IC50 of 5.45 and 9.47 μM, respectively. A molecular docking study with respect to COVID-19 main protease (Mpro) (PDB ID: 6LU7) has also been carried out which shows comparatively high binding affinity of compounds 3f and 3g (-8.3 and -8.8 Kcal/mole, respectively) than few reported drugs such as ritonavir, remdesivir, ribacvirin, favipiravir, hydroxychloroquine, chloroquine, and olsaltamivir. Hence, it reveals the possibility of these compounds to be used as effective COVID-19 inhibitors.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
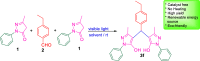


Similar articles
-
Synthesis of 4,4'-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) and evaluation of their antioxidant and anticancer activities.BMC Chem. 2021 Jun 3;15(1):38. doi: 10.1186/s13065-021-00765-y. BMC Chem. 2021. PMID: 34082794 Free PMC article.
-
Single crystal XRD, DFT investigations and molecular docking study of 2- ((1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)amino)naphthalene-1,4-dione as a potential anti- cancer lead molecule.Comput Biol Chem. 2019 Feb;78:153-164. doi: 10.1016/j.compbiolchem.2018.11.022. Epub 2018 Nov 30. Comput Biol Chem. 2019. PMID: 30530296
-
Visible-light promoted catalyst-free (VLCF) multi-component synthesis of spiro indolo-quinazolinone-pyrrolo[3,4-a]pyrrolizine hybrids: evaluation of in vitro anticancer activity, molecular docking, MD simulation and DFT studies.J Biomol Struct Dyn. 2024 Apr;42(6):3145-3165. doi: 10.1080/07391102.2023.2214229. Epub 2023 May 25. J Biomol Struct Dyn. 2024. PMID: 37227775
-
Nanofibrous matrixes with biologically active hydroxybenzophenazine pyrazolone compound for cancer theranostics.Mater Sci Eng C Mater Biol Appl. 2017 May 1;74:70-85. doi: 10.1016/j.msec.2017.01.001. Epub 2017 Feb 3. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 28254336
-
Poly(aniline-co-melamine)@MnFe2O4 nanocatalyst for the synthesis of 4,4'-(arylmethylene) bis (1H-pyrazole-5-ol) derivatives, and 1,4- dihydropyrano[2,3-c]pyrazoles and evaluation of their antioxidant, and anticancer activities.Front Chem. 2022 Oct 28;10:1046120. doi: 10.3389/fchem.2022.1046120. eCollection 2022. Front Chem. 2022. PMID: 36385997 Free PMC article.
Cited by
-
An ultrasound assisted, ionic liquid-molecular iodine synergy driven efficient green synthesis of pyrrolobenzodiazepine-triazole hybrids as potential anticancer agents.Front Pharmacol. 2023 May 5;14:1168566. doi: 10.3389/fphar.2023.1168566. eCollection 2023. Front Pharmacol. 2023. PMID: 37214464 Free PMC article.
-
Natural product-inspired synthesis of coumarin-chalcone hybrids as potential anti-breast cancer agents.Front Pharmacol. 2023 Sep 6;14:1231450. doi: 10.3389/fphar.2023.1231450. eCollection 2023. Front Pharmacol. 2023. PMID: 37745072 Free PMC article.
-
Extended insight into the catalytic activity of boron-doped graphitic carbon nitride for the synthesis of bis-pyrazolyl methanes and pyranopyrazoles.Sci Rep. 2025 Jul 26;15(1):27303. doi: 10.1038/s41598-025-11187-4. Sci Rep. 2025. PMID: 40715186 Free PMC article.
-
Novel modified magnetic graphene oxide as an efficient and green catalyst for one-pot synthesis of pyrazoles.Sci Rep. 2025 Apr 24;15(1):14263. doi: 10.1038/s41598-025-97884-6. Sci Rep. 2025. PMID: 40274851 Free PMC article.
-
A Comprehensive Update of Anti-COVID-19 Activity of Heterocyclic Compounds.Drug Des Devel Ther. 2024 May 8;18:1547-1571. doi: 10.2147/DDDT.S450499. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38737333 Free PMC article. Review.
References
-
- Prier C. K.; Rankic D. A.; MacMillan W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322.10.1021/cr300503r. - DOI - PMC - PubMed
- Xuan J.; Lu L.-Q.; Chen J.-R.; Xiao W.-J. Visible-Light-Driven Photoredox Catalysis in the Construction of Carbocyclic and Heterocyclic Ring Systems. Eur. J. Org. Chem. 2013, 2013, 6755.10.1002/ejoc.201300596. - DOI
- Chen J.-R.; Hu X.-Q.; Lu L.-Q.; Xiao W.-J. Visible Light Photoredox-controlled Reactions of N-radicals and Radical Ions. Chem. Soc. Rev. 2016, 45, 2044.10.1039/c5cs00655d. - DOI - PubMed
-
- Yoon T. P.; Ischay M. A.; Du J. Visible Light Photocatalysis as a Greener Approach to Photochemical Synthesis. Nat. Chem. 2010, 2, 527.10.1038/nchem.687. - DOI - PubMed
- Narayanam J. M. R.; Stephenson C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102.10.1039/b913880n. - DOI - PubMed
- Teplý F. Photoredox Catalysis by [Ru(bpy)3]2+ to Trigger Transformations of Organic Molecules. Organic Synthesis using Visible-Light Photocatalysis and its 20th Century Roots. Collect.Czech. Chem. Commun. 2011, 76, 859.10.1135/cccc2011078. - DOI
- Xuan J.; Xiao W.-J. Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2012, 51, 6828.10.1002/anie.201200223. - DOI - PubMed
- Shi L.; Xia W. Photoredox Functionalization of C–H Bonds Adjacent to a Nitrogen Atom. Chem. Soc. Rev. 2012, 41, 7687.10.1039/c2cs35203f. - DOI - PubMed
- Hari D. P.; König B. The Photocatalyzed Meerwein Arylation: Classic Reaction of Aryl Diazonium Salts in a New Light. Angew. Chem. Int. Ed. 2013, 52, 4734.The Photoredox-Catalyzed Meerwein Addition Reaction: Intermolecular Amino-Arylation of Alkenes. Angew. Chem. Int. Ed.,2014, 53, 72510.1002/anie.201210276. - DOI - PubMed
- Xi Y.; Yi H.; Lei A. Synthetic Applications of Photoredox Catalysis with Visible Light. Org. Biomol. Chem. 2013, 11, 2387.10.1039/c3ob40137e. - DOI - PubMed
- Dai X.-J.; Xu X.-L.; Li X.-N. Applications of Visible Light Photoredox Catalysis in Organic Synthesis. Chin. J. Org. Chem. 2013, 33, 2046.10.6023/cjoc201304026. - DOI
-
- Fagnoni M.; Dondi D.; Ravelli D.; Albini A. Photocatalysis for the Formation of the C-C Bond. Chem. Rev. 2007, 107, 2725.10.1021/cr068352x. - DOI - PubMed
- Gambarotti C.; Punta C.; Recupero F.; Caronna T.; Palmisano L. Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications. Curr. Org. Chem. 2010, 14, 1153.10.2174/138527210791317111. - DOI
- Zeitler K. Photoredox Catalysis with Visible Light. Angew. Chem. Int. Ed. 2009, 48, 9785.10.1002/anie.200904056. - DOI - PubMed
- Hering T.; Hari D. P.; König B. Visible-Light-Mediated α-Arylation of Enol Acetates Using Aryl Diazonium Salts. J. Org. Chem. 2012, 77, 10347.10.1021/jo301984p. - DOI - PubMed
- Rueping M.; Vila C.; Bootwicha T. Continuous Flow Organocatalytic C–H Functionalization and Cross-Dehydrogenative Coupling Reactions: Visible Light Organophotocatalysis for Multicomponent Reactions and C–C, C–P Bond Formations. ACS Catal 2013, 3, 1676.10.1021/cs400350j. - DOI
- Skubi K. L.; Yoon T. P. Shape Control in Reactions with Light. Nature 2014, 515, 45.10.1038/515045a. - DOI - PubMed
- Xu Z.; Gao L.; Wang L.; Gong M.; Wang W.; Yuan R. Visible Light Catalysis Assisted Site-Specific Functionalization of Amino Acid Derivatives by C–H Bond Activation without Oxidant: Cross-Coupling Hydrogen Evolution Reaction. ACS Catal 2015, 5, 2391.10.1021/cs5011037. - DOI
- Ravelli D.; Protti S.; Albini A. Energy and Molecules from Photochemical/Photocatalytic Reactions: An Overview. Molecules 2015, 20, 1527.10.3390/molecules20011527. - DOI - PMC - PubMed
-
- Reckenthäler M.; Griesbeck A. G. Photoredox Catalysis for Organic Syntheses. Adv. Synth. Catal. 2013, 355, 2727.10.1002/adsc.201300751. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

