Endovascular treatment of aneurysms of the paraophthalmic segment of the internal carotid artery: Current status
- PMID: 36188411
- PMCID: PMC9523143
- DOI: 10.3389/fneur.2022.913704
Endovascular treatment of aneurysms of the paraophthalmic segment of the internal carotid artery: Current status
Abstract
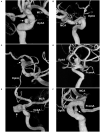
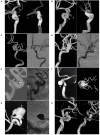
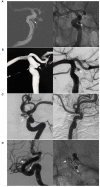
The paraophthalmic segment of the internal carotid artery (ICA) originates from the distal border of the cavernous ICA and terminates at the posterior communicating artery. Aneurysms arising from the paraophthalmic segment represent ~5-10% of intradural aneurysms. Due to the advent of endovascular treatment (EVT) techniques, specifically flow-diverting stents (FDSs), EVT has become a good option for these aneurysms. A literature review on EVT for paraophthalmic segment aneurysms is necessary. In this review, we discuss the anatomy of the paraophthalmic segment, classification of the paraophthalmic segment aneurysms, EVT principle and techniques, and prognosis and complications. EVT techniques for paraophthalmic segment aneurysms include coil embolization, FDSs, covered stents, and Woven EndoBridge devices. Currently, coiling embolization remains the best choice for ruptured paraophthalmic segment aneurysms, especially to avoid long-term antiplatelet therapy for young patients. Due to the excessive use of antiplatelet therapy, unruptured paraophthalmic segment aneurysms that are easy to coil should not be treated with FDS. FDS is appropriate for uncoilable or failed aneurysms. Other devices cannot act as the primary choice but can be useful auxiliary tools. Both coiling embolization and FDS deployment can result in a good prognosis for paraophthalmic segment aneurysms. The overall complication rate is low. Therefore, EVT offers promising treatments for paraophthalmic segment aneurysms. In addition, surgical clipping continues to be a good choice for paraophthalmic segment aneurysms in the endovascular era.
Keywords: aneurysm; endovascular treatment; internal carotid artery; paraophthalmic segment; review.
Copyright © 2022 Wang and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


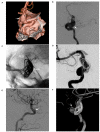
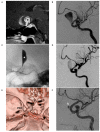
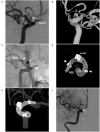
Similar articles
-
[Management of paraophthalmic aneurysms : Review of endovascular treatment strategies].Ophthalmologe. 2018 Feb;115(2):114-122. doi: 10.1007/s00347-017-0497-8. Ophthalmologe. 2018. PMID: 28439656 Review. German.
-
Endovascular treatment of intracranial internal carotid artery bifurcation region aneurysms.Front Neurol. 2024 Mar 28;15:1344388. doi: 10.3389/fneur.2024.1344388. eCollection 2024. Front Neurol. 2024. PMID: 38606281 Free PMC article. Review.
-
Endovascular treatment of middle cerebral artery aneurysms: current status and future prospects.Front Neurol. 2023 Nov 15;14:1239199. doi: 10.3389/fneur.2023.1239199. eCollection 2023. Front Neurol. 2023. PMID: 38033773 Free PMC article. Review.
-
Extending the Indication of Woven EndoBridge (WEB) Embolization to Internal Carotid Artery Aneurysms: A Multicenter Safety and Feasibility Study.World Neurosurg. 2019 Jun;126:e965-e974. doi: 10.1016/j.wneu.2019.02.198. Epub 2019 Mar 12. World Neurosurg. 2019. PMID: 30876989
-
Endovascular treatment strategies and a new classification for multiple aneurysms of the ipsilateral ophthalmic segment of the internal carotid artery.Asian J Surg. 2023 Sep;46(9):3663-3672. doi: 10.1016/j.asjsur.2023.03.134. Epub 2023 Apr 1. Asian J Surg. 2023. PMID: 37012159
Cited by
-
Application of the Willis Covered Stent in the Treatment of Complex Vascular Diseases of the Internal Carotid Artery and Vertebral Artery: A Retrospective Single-Center Experience.Ther Clin Risk Manag. 2023 Sep 25;19:773-782. doi: 10.2147/TCRM.S417803. eCollection 2023. Ther Clin Risk Manag. 2023. PMID: 37786750 Free PMC article.
-
Microsurgical treatment of ophthalmic artery aneurysm, a case series of 55 patients with long-term follow-up.BMC Surg. 2024 May 7;24(1):139. doi: 10.1186/s12893-024-02419-x. BMC Surg. 2024. PMID: 38714953 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

