The hunt for hidden hearing loss in humans: From preclinical studies to effective interventions
- PMID: 36188462
- PMCID: PMC9519997
- DOI: 10.3389/fnins.2022.1000304
The hunt for hidden hearing loss in humans: From preclinical studies to effective interventions
Abstract
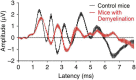
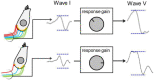
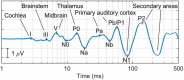
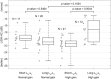
Many individuals experience hearing problems that are hidden under a normal audiogram. This not only impacts on individual sufferers, but also on clinicians who can offer little in the way of support. Animal studies using invasive methodologies have developed solid evidence for a range of pathologies underlying this hidden hearing loss (HHL), including cochlear synaptopathy, auditory nerve demyelination, elevated central gain, and neural mal-adaptation. Despite progress in pre-clinical models, evidence supporting the existence of HHL in humans remains inconclusive, and clinicians lack any non-invasive biomarkers sensitive to HHL, as well as a standardized protocol to manage hearing problems in the absence of elevated hearing thresholds. Here, we review animal models of HHL as well as the ongoing research for tools with which to diagnose and manage hearing difficulties associated with HHL. We also discuss new research opportunities facilitated by recent methodological tools that may overcome a series of barriers that have hampered meaningful progress in diagnosing and treating of HHL.
Keywords: central gain; cochlear synaptopathy; demyelination; hearables; hearing aids; noise exposure; noise-induced hearing loss; speech-in-noise hearing difficulties.
Copyright © 2022 Valderrama, de la Torre and McAlpine.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility.Hear Res. 2018 Aug;365:36-48. doi: 10.1016/j.heares.2018.06.003. Epub 2018 Jun 12. Hear Res. 2018. PMID: 29913342
-
Using Thresholds in Noise to Identify Hidden Hearing Loss in Humans.Ear Hear. 2018 Sep/Oct;39(5):829-844. doi: 10.1097/AUD.0000000000000543. Ear Hear. 2018. PMID: 29337760 Free PMC article.
-
Contrasting mechanisms for hidden hearing loss: Synaptopathy vs myelin defects.PLoS Comput Biol. 2021 Jan 22;17(1):e1008499. doi: 10.1371/journal.pcbi.1008499. eCollection 2021 Jan. PLoS Comput Biol. 2021. PMID: 33481777 Free PMC article.
-
Hidden hearing loss: current concepts.Curr Opin Otolaryngol Head Neck Surg. 2022 Oct 1;30(5):321-325. doi: 10.1097/MOO.0000000000000824. Epub 2022 Jul 5. Curr Opin Otolaryngol Head Neck Surg. 2022. PMID: 36004790 Review.
-
Noise-induced cochlear synaptopathy: Past findings and future studies.Hear Res. 2017 Jun;349:148-154. doi: 10.1016/j.heares.2016.12.008. Epub 2016 Dec 19. Hear Res. 2017. PMID: 28007526 Review.
Cited by
-
A genome-wide association study reveals a polygenic architecture of speech-in-noise deficits in individuals with self-reported normal hearing.Sci Rep. 2024 Jun 7;14(1):13089. doi: 10.1038/s41598-024-63972-2. Sci Rep. 2024. PMID: 38849415 Free PMC article.
-
Efficacy of Hearing Aids in Patients with Hearing Difficulties in Noise: Focus on Hidden Hearing Loss.J Clin Med. 2025 Jan 9;14(2):360. doi: 10.3390/jcm14020360. J Clin Med. 2025. PMID: 39860366 Free PMC article.
-
The role of GDF15 in attenuating noise-induced hidden hearing loss by alleviating oxidative stress.Cell Biol Toxicol. 2024 Sep 18;40(1):79. doi: 10.1007/s10565-024-09912-2. Cell Biol Toxicol. 2024. PMID: 39289208 Free PMC article.
-
ICF-based hearing and functioning assessment: validation and research outcomes of utilizing the HEAR-COMMAND tool for patients with mild to moderately severe hearing loss and individuals with normal hearing.Front Rehabil Sci. 2024 Aug 26;5:1389653. doi: 10.3389/fresc.2024.1389653. eCollection 2024. Front Rehabil Sci. 2024. PMID: 39253024 Free PMC article.
-
Predicting speech-in-noise ability in normal and impaired hearing based on auditory cognitive measures.Front Neurosci. 2023 Feb 7;17:1077344. doi: 10.3389/fnins.2023.1077344. eCollection 2023. Front Neurosci. 2023. PMID: 36824211 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

