Brain and brain-heart Granger causality during wakefulness and sleep
- PMID: 36188466
- PMCID: PMC9520578
- DOI: 10.3389/fnins.2022.927111
Brain and brain-heart Granger causality during wakefulness and sleep
Abstract
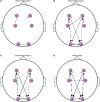
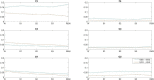
In this exploratory study we apply Granger Causality (GC) to investigate the brain-brain and brain-heart interactions during wakefulness and sleep. Our analysis includes electroencephalogram (EEG) and electrocardiogram (ECG) data during all-night polysomnographic recordings from volunteers with apnea, available from the Massachusetts General Hospital's Computational Clinical Neurophysiology Laboratory and the Clinical Data Animation Laboratory. The data is manually annotated by clinical staff at the MGH in 30 second contiguous intervals (wakefulness and sleep stages 1, 2, 3, and rapid eye movement (REM). We applied GC to 4-s non-overlapping segments of available EEG and ECG across all-night recordings of 50 randomly chosen patients. To identify differences in GC between the different sleep stages, the GC for each sleep stage was subtracted from the GC during wakefulness. Positive (negative) differences indicated that GC was greater (lower) during wakefulness compared to the specific sleep stage. The application of GC to study brain-brain and brain-heart bidirectional connections during wakefulness and sleep confirmed the importance of fronto-posterior connectivity during these two states, but has also revealed differences in ipsilateral and contralateral mechanisms of these connections. It has also confirmed the existence of bidirectional brain-heart connections that are more prominent in the direction from brain to heart. Our exploratory study has shown that GC can be successfully applied to sleep data analysis and captures the varying physiological mechanisms that are related to wakefulness and different sleep stages.
Keywords: Granger causality; connectivity; electrocardiogram (ECG); electroencephalogram (EEG); sleep.
Copyright © 2022 Abdalbari, Durrani, Pancholi, Patel, Nasuto and Nicolaou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Anzolin A., Presti P., Van de Steen F., Astolfi L., Haufe S., Marinazzo D. (2019). Quantifying the effect of demixing approaches on directed connectivity estimated between reconstructed EEG sources. Brain Topogr. Controv. EEG Source Imaging Connect. Model. Valid. Benchmark. 32 655–674. 10.1007/s10548-019-00705-z - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

