Absence of the Fragile X messenger ribonucleoprotein alters response patterns to sounds in the auditory midbrain
- PMID: 36188480
- PMCID: PMC9523263
- DOI: 10.3389/fnins.2022.987939
Absence of the Fragile X messenger ribonucleoprotein alters response patterns to sounds in the auditory midbrain
Abstract
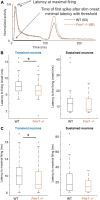
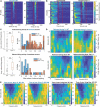
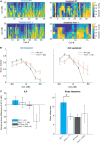
Among the different autism spectrum disorders, Fragile X syndrome (FXS) is the most common inherited cause of intellectual disability. Sensory and especially auditory hypersensitivity is a key symptom in patients, which is well mimicked in the Fmr1 -/- mouse model. However, the physiological mechanisms underlying FXS's acoustic hypersensitivity in particular remain poorly understood. Here, we categorized spike response patterns to pure tones of different frequencies and intensities from neurons in the inferior colliculus (IC), a central integrator in the ascending auditory pathway. Based on this categorization we analyzed differences in response patterns between IC neurons of wild-type (WT) and Fmr1 -/- mice. Our results report broadening of frequency tuning, an increased firing in response to monaural as well as binaural stimuli, an altered balance of excitation-inhibition, and reduced response latencies, all expected features of acoustic hypersensitivity. Furthermore, we noticed that all neuronal response types in Fmr1 -/- mice displayed enhanced offset-rebound activity outside their excitatory frequency response area. These results provide evidence that the loss of Fmr1 not only increases spike responses in IC neurons similar to auditory brainstem neurons, but also changes response patterns such as offset spiking. One can speculate this to be an underlying aspect of the receptive language problems associated with Fragile X syndrome.
Keywords: Fragile X syndrome; auditory hypersensitivity; auditory processing; autism spectrum disorder; inferior colliculus; spike pattern.
Copyright © 2022 Sibille, Kremkow and Koch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

