A new promising oncogenic target (p.C382R) for treatment with pemigatinib in patients with cholangiocarcinoma
- PMID: 36188486
- PMCID: PMC9520138
- DOI: 10.1177/17588359221125096
A new promising oncogenic target (p.C382R) for treatment with pemigatinib in patients with cholangiocarcinoma
Abstract
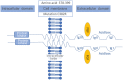
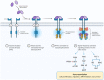
Point mutations of the fibroblast growth factor receptor (FGFR)2 receptor in intrahepatic cholangiocarcinoma (iCC) are mainly of unknown functional significance compared to FGFR2 fusions. Pemigatinib, a tyrosine kinase inhibitor, is approved for the treatment of cholangiocarcinoma with FGFR2 fusion/rearrangement. Although it is hypothesized that FGFR2 mutations may cause uncontrolled activation of the signaling pathway, the data for targeted therapies for FGFR2 mutations remain unclear. In vitro analyses demonstrated the importance of the p.C382R mutation for ligand-independent constitutive activation of FGFR2 with transforming potential. The following report describes the clinical case of a patient diagnosed with an iCC carrying a FGFR2 p.C382R point mutation which was detected in liquid, as well as in tissue-based biopsies. The patient was treated with pemigatinib, resulting in a sustained complete functional remission in fluorodeoxyglucose-positron emission tomography/computed tomography over 10 months to date. The reported case is the first description of a complete functional remission under the treatment with pemigatinib in a patient with p.C383R mutation.
Keywords: FGFR2; cholangiocarcinoma; mixed-all-nominated-in-one method; next-generation sequencing; targeted therapy; tyrosine kinase inhibitor.
© The Author(s), 2022.
Conflict of interest statement
Competing interests: The authors declare that there is no conflict of interest.
Figures





References
-
- Rizzo A, Ricci AD, Brandi G. Pemigatinib: hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat Res Commun 2021; 27: 100337. - PubMed
-
- Li F, Peiris MN, Donoghue DJ. Functions of FGFR2 corrupted by translocations in intrahepatic cholangiocarcinoma. Cytokine Growth Factor Rev 2020; 52: 56–67. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

