Electrosynthesis of ammonia with high selectivity and high rates via engineering of the solid-electrolyte interphase
- PMID: 36188748
- PMCID: PMC9511958
- DOI: 10.1016/j.joule.2022.07.009
Electrosynthesis of ammonia with high selectivity and high rates via engineering of the solid-electrolyte interphase
Abstract
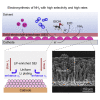
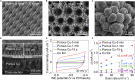
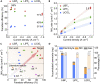
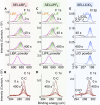
Ammonia is a large-scale commodity essential to fertilizer production, but the Haber-Bosch process leads to massive emissions of carbon dioxide. Electrochemical ammonia synthesis is an attractive alternative pathway, but the process is still limited by low ammonia production rate and faradaic efficiency. Herein, guided by our theoretical model, we present a highly efficient lithium-mediated process enabled by using different lithium salts, leading to the formation of a uniform solid-electrolyte interphase (SEI) layer on a porous copper electrode. The uniform lithium-fluoride-enriched SEI layer provides an ammonia production rate of 2.5 ± 0.1 μmol s-1 cmgeo -2 at a current density of -1 A cmgeo -2 with 71% ± 3% faradaic efficiency under 20 bar nitrogen. Experimental X-ray analysis reveals that the lithium tetrafluoroborate electrolyte induces the formation of a compact and uniform SEI layer, which facilitates homogeneous lithium plating, suppresses the undesired hydrogen evolution as well as electrolyte decomposition, and enhances the nitrogen reduction.
Keywords: electrochemical ammonia synthesis; high rates; high selectivity; lithium-mediated nitrogen reduction; solid-electrolyte interphase.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- United States Geological Survey (USGS) Mineral commodity summaries 2022: U.S.... nitrogen (fixed)–ammonia. 2022. https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-nitrogen.pdf
-
- MacFarlane D.R., Cherepanov P.V., Choi J., Suryanto B.H.R., Hodgetts R.Y., Bakker J.M., Ferrero Vallana F.M., Simonov A.N. A roadmap to the ammonia economy. Joule. 2020;4:1186–1205.
-
- Christensen C.H., Johannessen T., Sørensen R.Z., Nørskov J.K. Towards an ammonia-mediated hydrogen economy? Catal. Today. 2006;111:140–144.
-
- Haber F., Le Rossignol R. Über die technische Darstellung von Ammoniak aus den Elementen. Z. Elektrochem. Angew. Phys. Chem. 1913;19:53–72.
-
- Erisman J.W., Sutton M.A., Galloway J., Klimont Z., Winiwarter W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008;1:636–639.
LinkOut - more resources
Full Text Sources
Other Literature Sources
