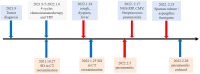
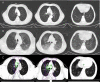
Case report: Pneumonia with clinical symptoms precedes imaging evidence after immune checkpoint inhibitors combined with radiotherapy in lung squamous cell cancer
- PMID: 36189237
- PMCID: PMC9520566
- DOI: 10.3389/fimmu.2022.998516
Case report: Pneumonia with clinical symptoms precedes imaging evidence after immune checkpoint inhibitors combined with radiotherapy in lung squamous cell cancer
Abstract
Immune-checkpoint inhibitors (ICI) targeting programmed cell death 1 (PD-1) and its ligand 1 (PD-L1) have quickly changed the treatment landscape in advanced non-small cell lung cancer. However, any patient treated with an immune checkpoint inhibitor is at risk for immune-related adverse events (irAEs). Checkpoint inhibitor pneumonitis (CIP) is a rare but potentially severe pulmonary toxicity of immunotherapy. Since the imaging features and symptoms are not specific, the diagnosis of CIP is challenging. In addition, CIP may mimic other lung diseases. Due to these characteristics, proper patient management may be delayed. So, a comprehensive understanding of imaging features is essential for a prompt detection and correct management of these drug-induced lung diseases. We presented a patient with lung squamous cell cancer who has clinical symptoms preceding imaging evidence of pneumonitis after immunotherapy and radiotherapy. We also discussed the safety of immunotherapy, the complexity and management of immune pneumonitis.
Keywords: cancer immunotherapy; immune checkpoint inhibitors (ICI); immune-related adverse event(irAE); pneumonitis; programmed cell death 1 inhibitor.
Copyright © 2022 Wang, Wang, Yu and Meng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Checkpoint Inhibitor Pneumonitis Induced by Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer: Occurrence and Mechanism.Front Immunol. 2022 Apr 7;13:830631. doi: 10.3389/fimmu.2022.830631. eCollection 2022. Front Immunol. 2022. PMID: 35464480 Free PMC article. Review.
-
Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: a multi-institutional cohort study.J Immunother Cancer. 2022 Jun;10(6):e004670. doi: 10.1136/jitc-2022-004670. J Immunother Cancer. 2022. PMID: 35705313 Free PMC article.
-
Risk Factors for Immune Checkpoint Inhibitor-Related Pneumonitis in Cancer Patients: A Systemic Review and Meta-Analysis.Respiration. 2022;101(11):1035-1050. doi: 10.1159/000526141. Epub 2022 Sep 15. Respiration. 2022. PMID: 36108598
-
Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: Case series and literature review.Thorac Cancer. 2020 Jul;11(7):1927-1933. doi: 10.1111/1759-7714.13483. Epub 2020 May 18. Thorac Cancer. 2020. PMID: 32421224 Free PMC article.
-
Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors.J Thorac Oncol. 2018 Dec;13(12):1930-1939. doi: 10.1016/j.jtho.2018.08.2035. Epub 2018 Sep 26. J Thorac Oncol. 2018. PMID: 30267842
Cited by
-
A Systematic Review of Pneumonitis Following Treatment with Immune Checkpoint Inhibitors and Radiotherapy.Biomedicines. 2025 Apr 12;13(4):946. doi: 10.3390/biomedicines13040946. Biomedicines. 2025. PMID: 40299683 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials