The short-term and long-term effects of intranasal mesenchymal stem cell administration to noninflamed mice lung
- PMID: 36189248
- PMCID: PMC9523259
- DOI: 10.3389/fimmu.2022.967487
The short-term and long-term effects of intranasal mesenchymal stem cell administration to noninflamed mice lung
Abstract
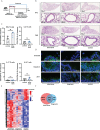
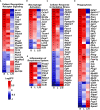
Mesenchymal stem cells (mesenchymal stromal cells; MSC)-based therapies remain a promising approach to treat degenerative and inflammatory diseases. Their beneficial effects were confirmed in numerous experimental models and clinical trials. However, safety issues concerning MSCs' stability and their long-term effects limit their implementation in clinical practice, including treatment of respiratory diseases such as asthma, chronic obstructive pulmonary disease, and COVID-19. Here, we aimed to investigate the safety of intranasal application of human adipose tissue-derived MSCs in a preclinical experimental mice model and elucidate their effects on the lungs. We assessed short-term (two days) and long-term (nine days) effects of MSCs administration on lung morphology, immune responses, epithelial barrier function, and transcriptomic profiles. We observed an increased frequency of IFNγ- producing T cells and a decrease in occludin and claudin 3 as a long-term effect of MSCs administration. We also found changes in the lung transcriptomic profiles, reflecting redox imbalance and hypoxia signaling pathway. Additionally, we found dysregulation in genes clustered in pattern recognition receptors, macrophage activation, oxidative stress, and phagocytosis. Our results suggest that i.n. MSCs administration to noninflamed healthy lungs induces, in the late stages, low-grade inflammatory responses aiming at the clearance of MSCs graft.
Keywords: epithelial barrier; mesenchymal stem cell; noninflamed lung; stem cell-based therapy; transcriptomic profiles.
Copyright © 2022 Tynecka, Janucik, Niemira, Zbikowski, Stocker, Tarasik, Starosz, Grubczak, Szalkowska, Korotko, Reszec, Kwasniewski, Kretowski, Akdis, Sokolowska, Moniuszko and Eljaszewicz.
Conflict of interest statement
MT reports grant from National Science Centre, Poland, during the conduct of the study, grants from European Union funds, POWER 2014-2020, grants from National Centre for Research and Development, outside the submitted work. AJ and AZ reports grants and non-financial support from Ministry of Education and Science, Poland. CA reports research grants from the Swiss National Science Foundation, European Union (EU CURE), Novartis Research Institutes (Basel, Switzerland), Stanford University (Redwood City, Calif), and SciBase (Stockholm, Sweden), he is the Co-Chair for EAACI Guidelines on Environmental Science in Allergic diseases and Asthma, and serves on the Advisory Boards of Sanofi/Regeneron, Novartis, GlaxoSmithKline, and SciBase, and is the Editor-in-Chief of Allergy, outside the submitted work. MS reports grants from Swiss National Science Foundation, grants from GSK, grants from Novartis, personal fees from AstraZeneca, outside the submitted work. MM reports grants from National Centre for Research and Development, grant from Medical Research Agency, lecture fees from Astra Zeneca, Berlin-Chemie/Menarini, GSK, Takeda, Shire, Teva, Lek-Am, Celon, Sandoz, Pfizer, Hal Allergy, and had reimbursed conference costs and travel by Berlin-Chemie/Menarini, outside the submitted work. AE reports grant from National Science Centre, during the conduct of the study, grants from National Centre for Research and Development. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Mesenchymal stem cell-based treatments for COVID-19: status and future perspectives for clinical applications.Cell Mol Life Sci. 2022 Feb 20;79(3):142. doi: 10.1007/s00018-021-04096-y. Cell Mol Life Sci. 2022. PMID: 35187617 Free PMC article. Review.
-
Current status of mesenchymal stem cell therapy for immune/inflammatory lung disorders: Gleaning insights for possible use in COVID-19.Stem Cells Transl Med. 2020 Oct;9(10):1163-1173. doi: 10.1002/sctm.20-0186. Epub 2020 Jun 11. Stem Cells Transl Med. 2020. PMID: 32526079 Free PMC article. Review.
-
Hypoxia-Induced Mesenchymal Stromal Cells Exhibit an Enhanced Therapeutic Effect on Radiation-Induced Lung Injury in Mice due to an Increased Proliferation Potential and Enhanced Antioxidant Ability.Cell Physiol Biochem. 2017;44(4):1295-1310. doi: 10.1159/000485490. Epub 2017 Nov 29. Cell Physiol Biochem. 2017. PMID: 29183009
-
Therapeutic administration of bone marrow-derived mesenchymal stromal cells reduces airway inflammation without up-regulating Tregs in experimental asthma.Clin Exp Allergy. 2018 Feb;48(2):205-216. doi: 10.1111/cea.13048. Epub 2017 Dec 15. Clin Exp Allergy. 2018. PMID: 29068567
-
Bone Marrow, Adipose, and Lung Tissue-Derived Murine Mesenchymal Stromal Cells Release Different Mediators and Differentially Affect Airway and Lung Parenchyma in Experimental Asthma.Stem Cells Transl Med. 2017 Jun;6(6):1557-1567. doi: 10.1002/sctm.16-0398. Epub 2017 Apr 20. Stem Cells Transl Med. 2017. PMID: 28425576 Free PMC article.
Cited by
-
Gene expression of protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1), solute carrier family 2 member 1 (SLC2A1) and mechanistic target of rapamycin (MTOR) in metformin-treated type 2 diabetes patients with COVID-19: impact on inflammation markers.Inflammopharmacology. 2024 Feb;32(1):885-891. doi: 10.1007/s10787-023-01341-7. Epub 2023 Sep 29. Inflammopharmacology. 2024. PMID: 37773574
-
Mesenchymal Stem/Stromal Cells in Asthma Therapy: Mechanisms and Strategies for Enhancement.Cell Transplant. 2023 Jan-Dec;32:9636897231180128. doi: 10.1177/09636897231180128. Cell Transplant. 2023. PMID: 37318186 Free PMC article. Review.
-
SARS-CoV-2 Spike Protein Induces Oxidative Stress and Senescence in Mouse and Human Lung.In Vivo. 2024 Jul-Aug;38(4):1546-1556. doi: 10.21873/invivo.13605. In Vivo. 2024. PMID: 38936937 Free PMC article.
-
The influence of asthmatic inflammation and house dust mite (HDM) exposure on abundance, immune-modulatory potential, and differentiation capacity of the lung-resident mesenchymal stem cells (lrMSCs).Stem Cell Res Ther. 2025 Jul 22;16(1):396. doi: 10.1186/s13287-025-04520-1. Stem Cell Res Ther. 2025. PMID: 40696474 Free PMC article.
References
-
- Wei Y, Chen X, Zhang H, Su Q, Peng Y, Fu Q, et al. . Efficacy and safety of bone marrow-derived mesenchymal stem cells for chronic antibody-mediated rejection after kidney transplantation- a single-arm, two-Dosing-Regimen, phase I/II study. Front Immunol (2021) 12:662441. doi: 10.3389/fimmu.2021.662441 - DOI - PMC - PubMed
-
- Piekarska K, Urban-Wójciuk Z, Kurkowiak M, Pelikant-Małecka I, Schumacher A, Sakowska J, et al. . Mesenchymal stem cells transfer mitochondria to allogeneic tregs in an HLA-dependent manner improving their immunosuppressive activity. Nat Commun (2022) 13(1):856. doi: 10.1038/s41467-022-28338-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

